Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a.\(n_{NaOH}=\dfrac{8}{40}=0,2mol\)
\(V_{dd}=\dfrac{120}{1,2}=100ml=0,1l\)
\(C_{M_{NaOH}}=\dfrac{0,2}{0,1}=2M\)
b.\(n_{NaOH}=\dfrac{21,6}{40}=0,54mol\)
\(V_{dd}=\dfrac{180}{1,2}=150ml=0,15l\)
\(C_{M_{NaOH}}=\dfrac{0,54}{0,15}=3,6M\)

\(Na+H_2O->NaOH+\dfrac{1}{2}H_2\\ a.n_{Na}=\dfrac{m_1}{23}\left(mol\right)\\ m_{ddsau}=\dfrac{m_1}{23}+m_2-\dfrac{m_1}{46}=\dfrac{m_1}{46}+m_2\left(g\right)\\ C\%_B=\dfrac{\dfrac{40}{23}m_1}{\dfrac{m_1}{46}+m_2}\cdot100\%.\\ b.C_M=\dfrac{10dC\%}{M}=10\cdot1,2\cdot\dfrac{0,05}{40}=0,015\left(M\right)\)
\(Na+H_2O->NaOH+\dfrac{1}{2}H_2\\ a.n_{Na}=\dfrac{m_1}{23}\left(mol\right)\\ m_{ddsau}=m_1+m_2-\dfrac{m_1}{23}=\dfrac{22}{23}m_1+m_2\left(g\right)\\ C\%_B=\dfrac{\dfrac{40}{23}m_1}{\dfrac{22}{23}m_1+m_2}\cdot100\%.\\ b.C_M=\dfrac{10dC\%}{M}=10\cdot1,2\cdot\dfrac{0,05}{40}=0,015\left(M\right)\)

1) Khối lượng mol của hợp chất:
\(M_{hc}=\dfrac{48}{20\%}=240\) (g/mol)
Khối lượng mol của 3 nguyên tử R:
\(3M_R=240-48=192\) (g/mol)
\(M_R=\dfrac{192}{3}=64\) (g/mol)
Vậy: R là Đồng (kí hiệu: Cu)
1)mO(hc)=n*M=3*16=48 (g)
⇒Mhc=mO/%mO*100=48/30*100=160 (g/mol)
Mhc=2*MR+3*MO
⇒160=2*MR+48⇒2*MR=160-48=112⇒MR=112/2=56 (g/mol)
⇒R là sắt (Fe)

a) \(n_{SO_3}=\dfrac{3,2}{80}=0,04\left(mol\right)\)
PTHH: SO3 + H2O --> H2SO4
0,04------------->0,04
=> \(m_{H_2SO_4}=0,04.98=3,92\left(g\right)\)
b) \(n_{Na}=\dfrac{0,69}{23}=0,03\left(mol\right)\)
PTHH: 2Na + 2H2O --> 2NaOH + H2
0,03------------>0,03
2NaOH + H2SO4 --> Na2SO4 + 2H2O
Xét tỉ lệ: \(\dfrac{0,03}{2}< \dfrac{0,04}{1}\)=> NaOH hết, H2SO4 dư
2NaOH + H2SO4 --> Na2SO4 + 2H2O
0,03------>0,015---->0,015
\(\left\{{}\begin{matrix}n_{Na_2SO_4}=0,015\left(mol\right)\\n_{H_2SO_4\left(dư\right)}=0,025\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}m_{Na_2SO_4}=0,015.142=2,13\left(g\right)\\m_{H_2SO_4}=0,025.98=2,45\left(g\right)\end{matrix}\right.\)
c) \(n_{Na}=\dfrac{2,07}{23}=0,09\left(mol\right)\)
PTHH: 2Na + 2H2O --> 2NaOH + H2
0,09-------------->0,09
Xét tỉ lệ: \(\dfrac{0,09}{2}>\dfrac{0,04}{1}\) => NaOH dư, H2SO4 hết
2NaOH + H2SO4 --> Na2SO4 + 2H2O
0,08<-----0,04------>0,04
=> \(\left\{{}\begin{matrix}n_{NaOH\left(dư\right)}=0,01\left(mol\right)\\n_{Na_2SO_4}=0,04\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}m_{NaOH\left(dư\right)}=0,01.40=0,4\left(g\right)\\m_{Na_2SO_4}=0,04.142=5,68\left(g\right)\end{matrix}\right.\)

\(C\%=\dfrac{30}{170}.100\%=17,647\%\)
\(V_{\text{dd}}=\left(30+170\right)1,1=220ml\)
\(n_{NaCl}=\dfrac{30}{58,5}=0,513mol\)
\(C_M=\dfrac{0,513}{0,22}=0,696M\)
\(C\%_{NaCl}=\dfrac{30}{170+30}.100\%=15\%\\ C_M=C\%.\dfrac{10D}{M}=10.\dfrac{10.1,1}{58,5}=1,88M\)

\(n_{Na_2CO_3}=\dfrac{10,6}{106}=0,1\left(mol\right)\\ \rightarrow C_{M\left(Na_2CO_3\right)}=\dfrac{0,1}{0,2}=0,5M\)
Ta có: \(C\%=\dfrac{C_M.M}{10.D}\)
\(\rightarrow C\%=\dfrac{0,5.106}{10.1,05}=5,05\%\)

\(n_K=\dfrac{3.9}{39}=0.1\left(mol\right)\)
\(K+H_2O\rightarrow KOH+\dfrac{1}{2}H_2\)
\(0.1...................0.1.....0.05\)
\(m_{H_2O}=26.2\cdot1=26.2\left(g\right)\)
\(m_{KOH}=0.1\cdot56=5.6\left(g\right)\)
\(m_{\text{dung dịch sau phản ứng}}=m_K+m_{H_2O}-m_{H_2}=3.9+26.2-0.05\cdot2=30\left(g\right)\)
\(C\%_{KOH}=\dfrac{5.6}{30}\cdot100\%=18.67\%\)
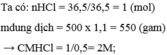
2) nNa=0,1(mol)
PTHH: Na + H2O -> NaOH + 1/2 H2
0,1_____________0,1_______0,05(mol)
- Chất tan: NaOH
mddNaOH= mNa+ mH2O - mH2= 2,3+100-0,05.2=102,2(g)
1) mC2H5OH=0,8.10=8(g)
mH2O=100.1=100(g)
mddC2H5OH=100+8=108(g)