Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

(1) 2Na+2H2O--->2NaOH+H2
(2) 3NaOH+Al(NO3)3--->Al(OH)3+3NaNO3
(3) 2Al(OH)3--->Al2O3+3H2O
(4) 2Al2O3-đpnc---> 4Al+3O2
(5) 2Al+3H2SO4--->Al2(SO4)3+3H2
(6) Al2(SO4)3+3BaCl2--->3BaSO4+2AlCl3

g) 1. Fe2O3 + 6HCl → 2FeCl3 + 3H2O
2. FeCl3 + 3NaOH → 3NaCl + Fe(OH)3↓
3. 2Fe(OH)3 \(\underrightarrow{to}\) Fe2O3 + 3H2O
4. Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O
h) 1. ZnCl2 + 2NaOH → 2NaCl + Zn(OH)2↓
2. Zn(OH)2 + 2HCl → ZnCl2 + 2H2O
3. ZnCl2 + 2NaOH → 2NaCl + Zn(OH)2↓
4. NaCl + AgNO3 → NaNO3 + AgCl↓

\(1\\ 2Al\left(OH\right)_3\rightarrow\left(t^o\right)Al_2O_3+3H_2O\\ 2\\ Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\\ Al_2\left(SO_4\right)_3+3BaCl_2\rightarrow3BaSO_4+2AlCl_3\)

(1) 2Fe + 3Cl2 -t0-> 2FeCl3
(2) FeCl3 + 3NaOH - > Fe(OH)3\(\downarrow\) + 3NaCl
(3) \(2Fe\left(OH\right)3-^{t0}->Fe2O3+3H2O\)
(4) \(Fe2O3+3H2SO4->Fe2\left(SO4\right)3+3H2O\)

(1) S+02===> S02 ( Nhiệt độ)
(2) 2SO2+ 02===> 2S03( ĐIỀU KIỆN V2O5, 450 độ)
(3) S03+ H20=====> H2S04
(4)3 H2S04+ 2Al====> Al2( S04)3+ 3/2H2
(5) ZnO+ H2===> Zn+ H20
(6) Zn+ HCl=====> ZnCl2+ H2
theo tớ nghĩ thôi nhá

a)\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
\(2AlCl_3+3Ba\left(OH\right)_2\rightarrow2Al\left(OH\right)_3\downarrow+3BaCl_2\)
\(2Al\left(OH\right)_3\underrightarrow{t^o}Al_2O_3+3H_2O\)
\(2Al+3S\underrightarrow{t^o}Al_2S_3\)

`(1)FeO + H_2SO_4 -> FeSO_4 + H_2O`
`(2)FeSO_4 + Ba(OH)_2 -> Fe(OH)_2 + BaSO_4`
`(3)Fe(OH)_2 -> (t^o, chân.không) FeO + H_2O`
`(4)FeO + 2HCl -> FeCl_2 + H_2O`
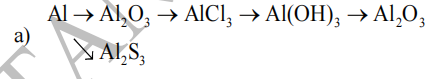
1) Al\(_2\)(SO\(_4\))\(_3\)+3BaCl\(_2\)→2AlCl\(_3\)+3BaSO\(_4\)↓
2)AlCl\(_3\)+3AgNO\(_3\)→Al(NO\(_3\))\(_3\)+3AgCl↓
3)Al(NO\(_3\))\(_3\)+3NaOH→Al(OH)\(_3\)+3NaNO\(_3\)
4)2Al(OH)\(_3\)\(\underrightarrow{to}\)Al2O3+3H2O
5)2Al2O3\(\xrightarrow[criolit]{đpnc}\)4Al+3O2