Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(2Al+3H_2SO_4\to Al_2(SO_4)_3+3H_2\\ Al_2(SO_4)_3+3BaCl_2\to 2AlCl_3+3BaSO_4\downarrow\\ AlCl_3+3NaOH\to Al(OH)_3\downarrow +3NaCl\\ 2Al(OH)_3\xrightarrow{t^o}Al_2O_3+3H_2O\\ 2Al_2O_3\xrightarrow{đpnc}4Al+3O_2\)

(1) \(4Al+3O_2-t^o-2Al_2O_3\)
(2) \(Al_2O_3+6HCl-->2AlCl_3+3H_2O\)
(3) \(AlCl_3+3AgNO_3-->3AgCl\downarrow+Al\left(NO_3\right)_3\)
(4) \(Al\left(NO_3\right)_3+3NaOH-->3NaNO_3+Al\left(OH\right)_3\downarrow\)

Bs đề câu a: \(Fe_2O_3\xrightarrow{(4)}Fe\)
\(a,(1)2Fe+3Cl_2\xrightarrow{t^o}2FeCl_3\\ (2)FeCl_3+3NaOH\to Fe(OH)_3\downarrow+3NaCl\\ (3)2Fe(OH)_3\xrightarrow{t^o}Fe_2O_3+3H_2O\\ (4)Fe_2O_3+3CO\xrightarrow{t^o}2Fe+3CO_2\)
\(b,(1)4Al+3O_2\xrightarrow{t^o}2Al_2O_3\\ (2)Al_2O_3+6HCl\to 2AlCl_3+3H_2\\ (3)AlCl_3+3NaOH\to Al(OH)_3\downarrow+3NaCl\\ (4)2Al(OH)_3\xrightarrow{t^o}Al_2O_3+3H_2O\)

\(4FeS_2+11O_2\rightarrow2Fe_2O_3+8SO_2\)
\(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\)
\(FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3+3NaCl\)
\(2Fe\left(OH\right)_3\rightarrow Fe_2O_3+3H_2O\)
\(H_2O+BaO\rightarrow Ba\left(OH\right)_2\)

\(4Al+3O_2\underrightarrow{^{^{t^o}}}2Al_2O_3\)
\(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\)
\(Al_2\left(SO_4\right)_3+6NaOH\rightarrow2Al\left(OH\right)_3+3Na_2SO_4\)
\(Al\left(OH\right)_3+3HCl\rightarrow AlCl_3+3H_2O\)
\(4Al+3O_2--^{t^o}->2Al_2O_3\)
\(Al_2O_3+3H_2SO_4--->Al_2\left(SO_4\right)_3+3H_2O\)
\(Al_2\left(SO_4\right)_3+3Cu\left(OH\right)_2--->2Al\left(OH\right)_3+3CuSO_4\)
\(Al\left(OH\right)_3+3HCl---->AlCl_3+3H_2O\)

a)
\(Al+\dfrac{3}{2}Cl_2\rightarrow AlCl_3\\ AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\\ 2Al\left(OH\right)_3\underrightarrow{t^o}Al_2O_3+3H_2O\\ Al_2O_3\xrightarrow[criolic]{đpnc}2Al+\dfrac{3}{2}O_2\)
b)
\(Fe+\dfrac{3}{2}Cl_2\rightarrow FeCl_3\\ FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3+3NaCl\\ 2Fe\left(OH\right)_3\underrightarrow{t^o}Fe_2O_3+3H_2O\\ Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
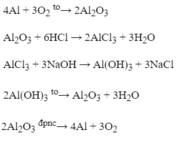
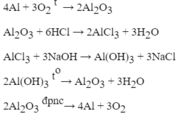
\(Ba\left(OH\right)_2+2HCl\rightarrow BaCl_2+2H_2O\\3 BaCl_2+Al_2\left(SO_4\right)_3\rightarrow3BaSO_4+2AlCl_3\\ AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\\ 2Al\left(OH\right)_3-^{t^o}\rightarrow Al_2O_3+3H_2O\\ 2Al_2O_3-^{đpnc,criolit}\rightarrow4Al+3O_2\)