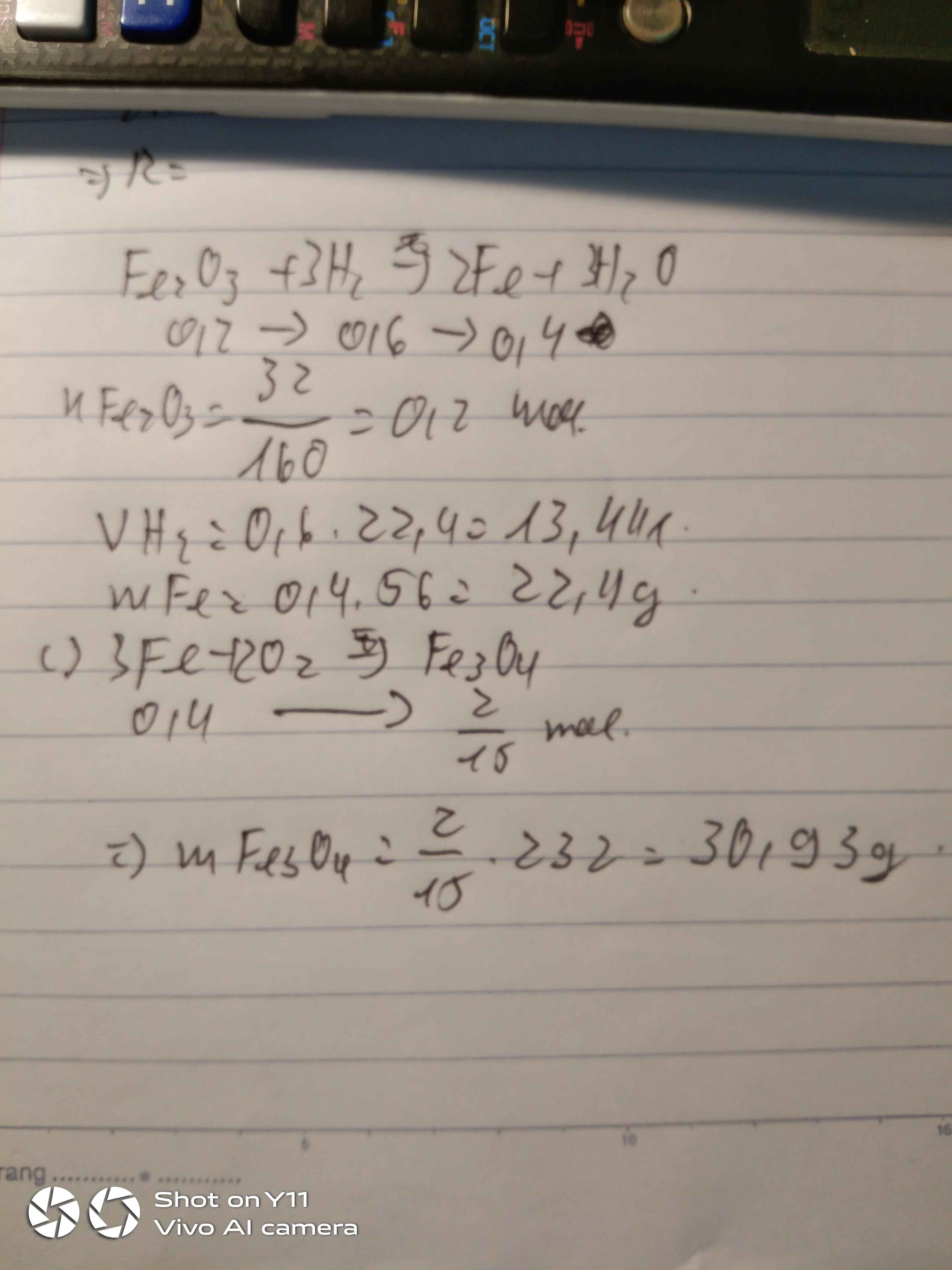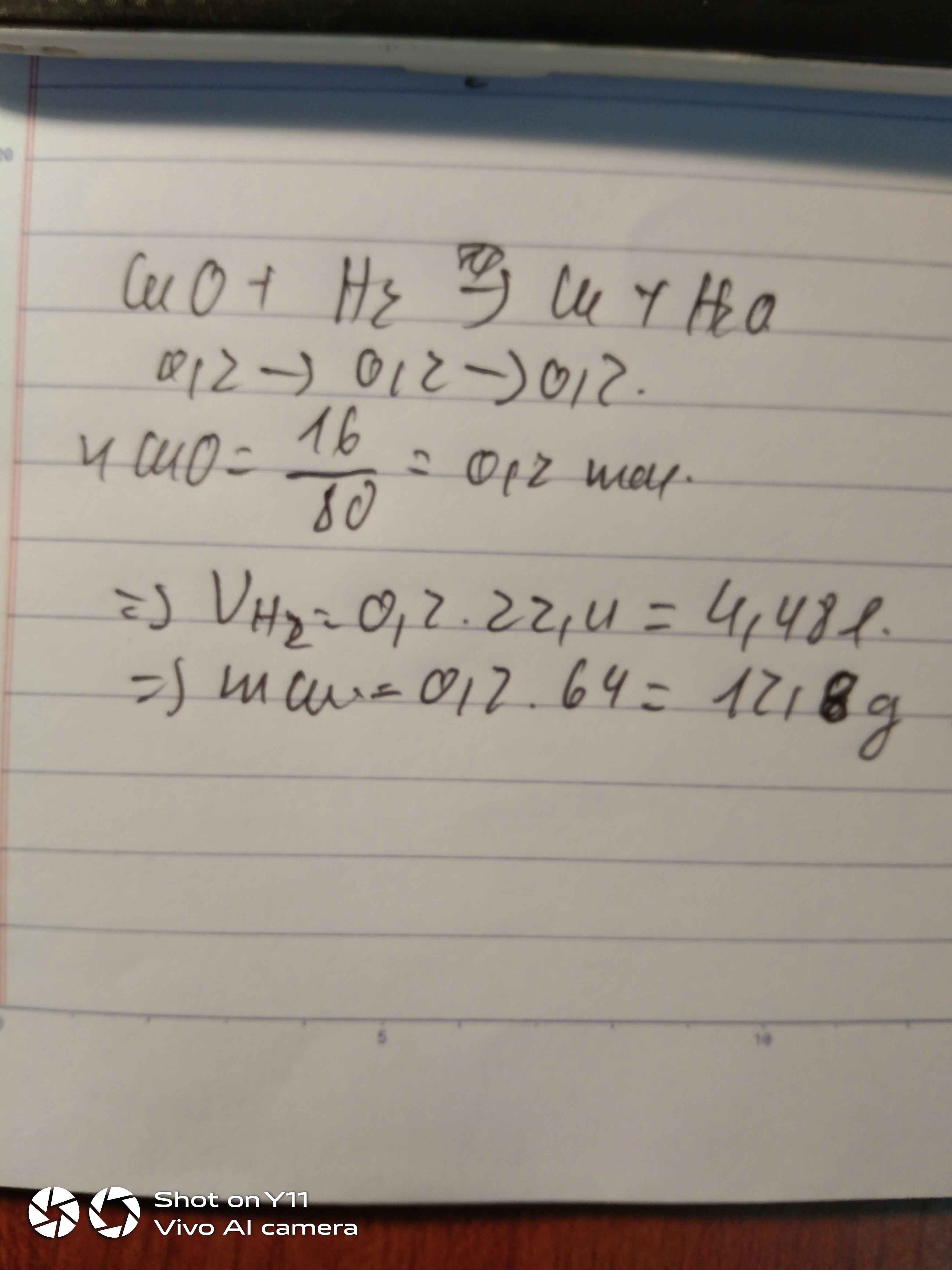Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{Zn}=\dfrac{9,75}{65}=0,15\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,15-->0,3------>0,15-->0,15
=> mHCl = 0,3.36,5 = 10,95 (g)
b)
mZnCl2 = 0,15.136 = 20,4 (g)
c)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,05<---0,15------->0,1
=> mFe2O3 = 0,05.160 = 8 (g)
mFe = 0,1.56 = 5,6 (g)
a.b.\(n_{Zn}=\dfrac{m_{Zn}}{M_{Zn}}=\dfrac{9,75}{65}=0,15mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,15 0,3 0,15 0,15 ( mol )
\(m_{HCl}=n_{HCl}.M_{HCl}=0,3.36,5=10,95g\)
\(m_{ZnCl_2}=n_{ZnCl_2}.M_{ZnCl_2}=0,15.136-20,4g\)
c.\(Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
0,05 0,15 0,1 ( mol )
\(m_{Fe_2O_3}=n_{Fe_2O_3}.M_{Fe_2O_3}=0,05.160=8g\)
\(m_{Fe}=n_{Fe}.M_{Fe}=0,1.56=5,6g\)

a. \(n_{O_2}=\dfrac{3.36}{22,4}=0,15\left(mol\right)\)
PTHH : 2Zn + O2 -------to------> 2ZnO
0,3 0,15 0,15
\(m_{Zn}=65.0,3=19,5\left(g\right)\)
b. \(m_{ZnO}=0,15.81=12,15\left(g\right)\)
c. PTHH : 2KMnO4 ---to---> K2MnO4 + MnO2 + O2
0,3 0,15
\(m_{KMnO_4}=158.0,3=47,4\left(g\right)\)
\(n_{O_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)\)
PTHH: 2Zn + O2 --to--> 2ZnO
3 1,5 3
\(\rightarrow\left\{{}\begin{matrix}m_{Zn}=3.65=195\left(g\right)\\m_{ZnO}=81.3=243\left(g\right)\end{matrix}\right.\)
2KMnO4 --to--> K2MnO4 + MnO2 + O2
6 3
=> mKMnO4 = 6.158 = 948 (g)

\(n_{HCl}=0,5.0,2=0,1\left(mol\right)\)
Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,05<--0,1----->0,05--->0,05
\(m_{Fe}=0,05.56=2,8\left(g\right)\)
\(m_{FeCl2}=0,05.127=6,35\left(g\right)\)
\(V_{H2\left(dktc\right)}=0,05.22,4=1,12\left(l\right)\)
\(V_{H2\left(dkc\right)}=0,05.24,79=1,2395\left(l\right)\)

\(n_{H_2}=\dfrac{2,9748}{24,79}=0,12(mol)\\ 2AL+6HCl\to 2AlCl_3+3H_2\\ \Rightarrow n_{Al}=n_{AlCl_3}=0,08(mol);n_{HCl}=0,24(mol)\\ a,m_{Al}=0,08.2=2,16(g)\\ m_{HCl}=0,24.36,5=8,76(g)\\ b,m_{AlCl_3}=0,08.133,5=10,68(g)\\ c,2H_2+O_2\xrightarrow{t^o}2H_2O\\ \Rightarrow n_{H_2O}=0,12(mol)\\ \Rightarrow m_{H_2O}=0,12.18=2,16(g)\)

nO2 = 3,36 : 22,4 = 0,15 (mol)
pthh : 2Mg + O2 -t--> 2MgO
0,3<----0,15---> 0,3 (mol)
=> mMg= 0,3 . 24 = 7,2 (g)
=> mMgO = 0,3 . 40 =12 (g)
pthh : 2KMnO4 -t--> K2MnO4 + MnO2 + O2
0,3<-------------------------------------0,15 (mol)
=> mKMnO4 = 0,3 . 158 = 47,4 (g)

a. \(n_{Cu}=\dfrac{28.8}{64}=0,45\left(mol\right)\)
PTHH : CuO + H2 -> Cu + H2O
0,45 0,45 0,45 0,45
\(V_{H_2}=0,45.22,4=10,08\left(l\right)\)
b. \(m_{Cu}=0,45.64=28,8\left(g\right)\)
ncu = 28,8/64 = 0,45 mol
CuO + H2 -> Cu + H2O
1 : 1 : 1 : 1
0,45mol
a) nH2 = (0,45.1) : 1 = 0,45 mol
VH2 = 0,45 . 22,4 = 10,08 ( l )
b) mCu = 0,45 . 64 = 28,8 ( g)

a, Ta có: \(n_{Fe}=\dfrac{33,6}{56}=0,6\left(mol\right)\)
\(n_{O_2}=\dfrac{12,395}{24,79}=0,5\left(mol\right)\)
PT: \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
Xét tỉ lệ: \(\dfrac{0,6}{3}< \dfrac{0,5}{2}\), ta được O2 dư.
Theo PT: \(n_{Fe_3O_3}=\dfrac{1}{3}n_{Fe}=0,2\left(mol\right)\)
\(\Rightarrow m_{Fe_3O_4}=0,2.232=46,4\left(g\right)\)
b, \(n_{H_2}=\dfrac{5,4198.10^{23}}{6,022.10^{23}}=0,9\left(mol\right)\)
PT: \(Fe_3O_4+4H_2\underrightarrow{t^o}3Fe+4H_2O\)
Xét tỉ lệ: \(\dfrac{0,2}{1}< \dfrac{0,9}{4}\), ta được H2 dư.
Theo PT: \(n_{Fe}=3n_{Fe_3O_4}=0,6\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,6.56=33,6\left(g\right)\)