Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{Cu}=\dfrac{19,2}{64}=0,3\left(mol\right)\)
PTHH: 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O
Mol: 0,3 0,8 0,2
\(V_{NO}=0,2.22,4=4,48\left(l\right)\)
b, \(C_{M_{ddHNO_3}}=\dfrac{0,8}{0,4}=2M\)

\(3Ag+4HNO_3\rightarrow3AgNO_3+NO+2H_2O\)
\(Al+4HNO_3\rightarrow Al\left(NO_3\right)_3+NO+2H_2O\)
\(NO\) là sản phẩm khử duy nhất.
\(\Rightarrow n_{NO}=\dfrac{0,448}{22,4}=0,02mol\)
Ta có: \(\left\{{}\begin{matrix}27n_{Al}+108n_{Ag}=3,51g\\BTe:3n_{Al}+n_{Ag}=3n_{NO}=0,06\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Al}=0,01mol\\n_{Ag}=0,03mol\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{0,01\cdot27}{3,51}\cdot100\%=7,7\%\)
\(\%m_{Ag}=100\%-7,7\%=92,3\%\)

Đáp án A
nNO = 2,8/22,4 = 0,125 (mol)
BTNT N: nNO3 ( trong muối) = 3nNO = 0,375 (mol)
=> mmuối = mKL + mNO3- = 7,55 + 0,375.62 = 30,8 (g)

\(\text{Đ}\text{ặt}:n_{Al}=a\left(mol\right);n_{Cu}=b\left(mol\right)\left(a,b>0\right)\\ Al+6HNO_3\rightarrow Al\left(NO_3\right)_3+3NO_2+3H_2O\\ Cu+4HNO_3\rightarrow Cu\left(NO_3\right)_2+2NO_2+2H_2O\\ \Rightarrow\left\{{}\begin{matrix}27a+64b=7,75\\3.22,4a+2.22,4b=7,84\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,05\\b=0,1\end{matrix}\right.\\ a,\Rightarrow\%m_{Al}=\dfrac{0,05.27}{7,75}.100\approx17,419\%\\ \Rightarrow\%m_{Cu}\approx82,581\%\\ b,n_{HNO_3}=6a+4b=0,7\left(mol\right)\\ C_{M\text{dd}HNO_3}=\dfrac{0,7}{0,14}=5\left(M\right)\)

- Viết đúng ptpư:
\(Fe+4HNO_3\rightarrow Fe\left(NO_3\right)_3+NO+2H_2O\)
\(3Cu+8HNO_3\rightarrow2Cu\left(NO_3\right)_2+2NO+4H_2O\)
\(nNO=0,04\left(mol\right)\)
Gọi nFe là x(mol) ; nCu là y(mol)
ta có hệ pt:
\(\left\{{}\begin{matrix}m_{hh}=56x+64y=3,04\\nNO=x+\dfrac{2}{3y}=0,04\end{matrix}\right.\)
Giải hệ ta được: x = 0,02 mol ; y = 0,03 mol
\(\Rightarrow mFe=0,02.56=1,12\left(g\right)\)
\(mCu=0,03.64=1,92\left(g\right)\)

a) nMg:a(mol) ,nAl:b(mol)
nNO=2,464/22,4=0,11(mol)
hpt: mX=24a+27b=3,42
nNO=23a+b=0,11
→a=0,075(mol),b=0,06(mol)
%mMg=(0,075.24/3,42).100%=52,63%
%mAl=100%−52,63%=47,37%
b)
nHNO3=4nNO=0,44(mol)
mdd HNO3=(0,44.63)/10%=277,2(g)
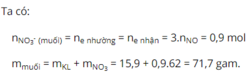

\(n_{NO}=0,28mol\)
\(\left\{{}\begin{matrix}BTe:3n_{Al}+2n_{Cu}=3n_{NO}=0,84\\27n_{Al}+64n_{Cu}=15,84\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Al}=0,16mol\\n_{Cu}=0,18mol\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{0,16\cdot27}{15,84}\cdot100=27,27\%\)
\(\Rightarrow\%m_{Cu}=100\%-27,27\%=72,73\%\)