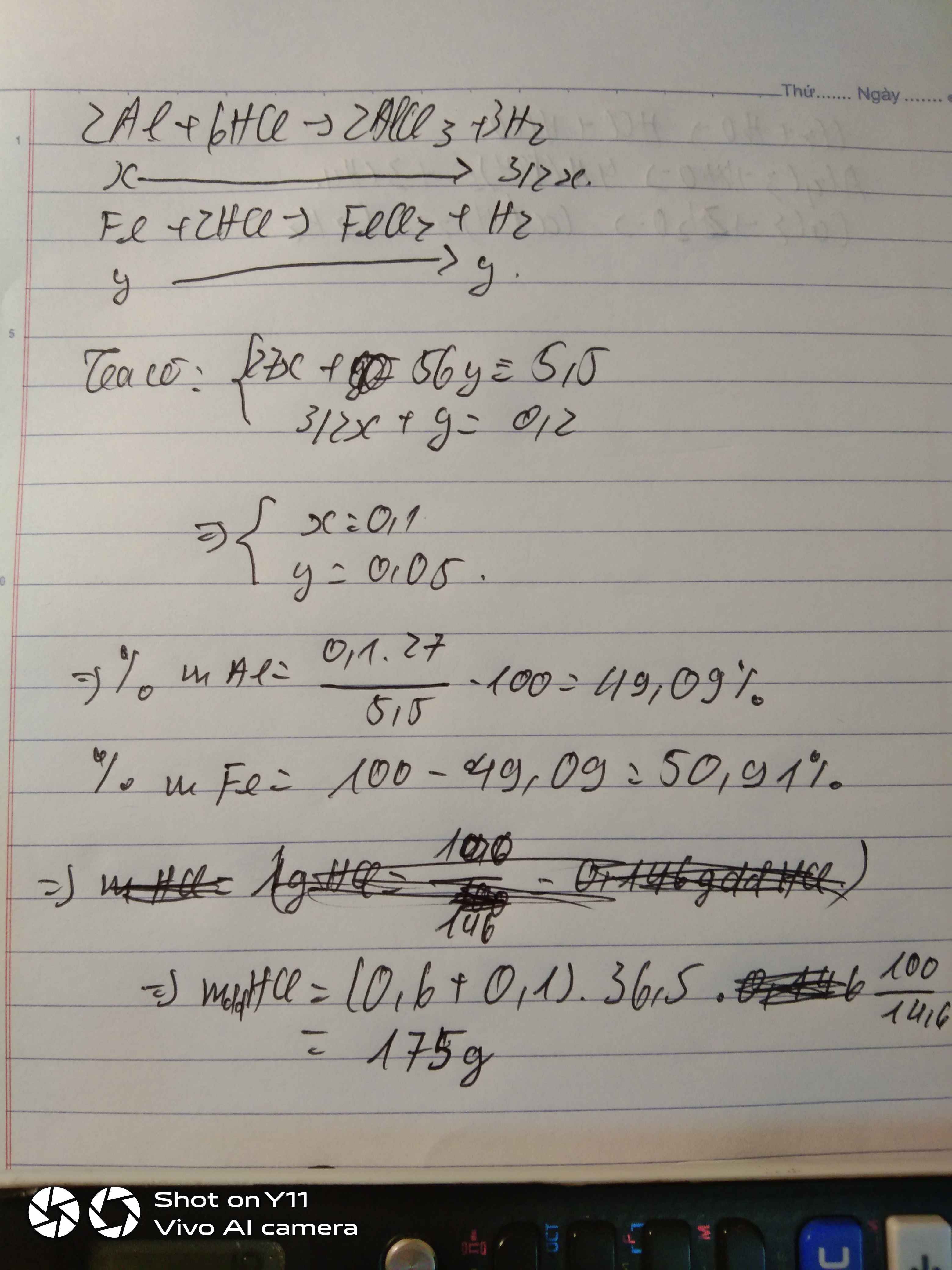Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: Mg +2 HCl -> MgCl2 + H2
x_________2x_____x_______x(mol)
Zn + 2 HCl -> ZnCl2 + H2
y___2y_____y_______y(mol)
Ta có hpt: \(\left\{{}\begin{matrix}24x+65y=15,3\\x+y=0,3\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=\dfrac{21}{205}\\y=\dfrac{81}{410}\end{matrix}\right.\)
=>mMg=21/205 . 24 = 504/205(g)
mZn=81/410 . 65=1053/82(g)

m(rắn)= mAg=3,2(g)
Fe +2 HCl -> FeCl2 + H2
nH2= 0,3(mol) -> nFe=0,3(mol)
=> mFe=0,3. 56=16,8(g)
=> m(hỗn hợp)= mAg+ mFe= 3,2+16,8=20(g)
=> %mAg= (3,2/20).100=16%
=>%mFe=100% - 16%=84%

Gọi \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo pt: \(\Rightarrow\left\{{}\begin{matrix}3x+y=0,2\\27x+56y=5,5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=\dfrac{19}{470}\\y=\dfrac{37}{470}\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{5,5}\cdot100\%=19,84\%\)
\(\%m_{Fe}=100\%-19,84\%=80,16\%\)

Gọi: \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\) ⇒ 27x + 56y = 5,5 (1)
PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Al}+n_{Fe}=\dfrac{3}{2}x+y=\dfrac{4,48}{22,4}=0,2\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,1\left(mol\right)\\y=0,05\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{5,5}.100\%\approx49,09\%\\\%m_{Fe}\approx50,91\%\end{matrix}\right.\)

A: MgO, CuO
B: MgCl2, CuCl2
C: Mg(OH)2, Cu(OH)2
PT: \(2Mg+O_2\underrightarrow{t^o}2MgO\)
\(2Cu+O_2\underrightarrow{t^o}2CuO\)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(MgCl_2+2NaOH\rightarrow2NaCl+Mg\left(OH\right)_{2\downarrow}\)
\(CuCl_2+2NaOH\rightarrow2NaCl+Cu\left(OH\right)_{2\downarrow}\)
\(Mg\left(OH\right)_2\underrightarrow{t^o}MgO+H_2O\)
\(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)

nH2= 0,15(mol)
PTHH: Mg + 2 HCl -> MgCl2 + H2
x______2x_______x________x(mol)
Fe+ 2 HCl ->FeCl2 + H2
y____2y______y___y(mol)
Ta có hpt: \(\left\{{}\begin{matrix}24x+56y=5,2\\x+y=0,15\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,05\end{matrix}\right.\)
mMg=0,1.24=2,4(g)
=> \(\%mMg=\dfrac{2,4}{5,2}.100\approx46,154\%\\ \Rightarrow\%mFe\approx53,846\%\)

nH2 = 13,44/22,4 = 0,6 (mol)
PTHH: Mg + 2HCl -> MgCl2 + H2
nHCl = 0,6 . 2 = 1,2 (mol)
mHCl = 1,2 . 36,5 = 43,8 (g)
nMg = 0,6 (mol)
mMg = 0,6 . 24 = 14,4 (g)
Không thấy mhh để tính%