
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.




PTHH: \(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\(Cu+2H_2SO_{4\left(đ\right)}\xrightarrow[]{t^o}CuSO_4+SO_2\uparrow+2H_2O\)
Khí A là SO2
Ta có: \(n_{SO_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)=n_{Cu}\)
\(\Rightarrow\%m_{Cu}=\dfrac{0,05\cdot64}{10}\cdot100\%=32\%\) \(\Rightarrow\%m_{CuO}=68\%\)

\(\left\{{}\begin{matrix}n_{Mg}=a\left(mol\right)\\n_{MgO}=b\left(mol\right)\end{matrix}\right.\left(a,b>0\right)\\ a.PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\\ a.......................2a.........a..........a\left(mol\right)\\ PTHH:MgO+2HCl\rightarrow MgCl_2+H_2O\\ b.....................2b.............b..............b\left(mol\right)\\ \rightarrow\left\{{}\begin{matrix}95a+95b=47,5\\a=0,2\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\\b=0,3\end{matrix}\right.\\ \rightarrow\left\{{}\begin{matrix}m_{Mg}=0,2.24=4,8\left(g\right)\\m_{MgO}=0,3.40=12\left(g\right)\end{matrix}\right.\\ \)
\(b.m_{HCl}=\left(2a+2b\right).36,5=36,5\left(g\right)\\ m_{ddHCl}=\dfrac{36,5.100}{14,6}=250\left(g\right)\\ m_{ddsau}=250+4,8+12-0,2.2=266,4\left(g\right)\\ C_{\%ddMgCl_2}=\dfrac{47,5}{266,4}.100\approx17,83\%\)

1/ Gọi x là hóa trị của nguyên tố cần tìm. Áp dụng quy tắc hóa trị:
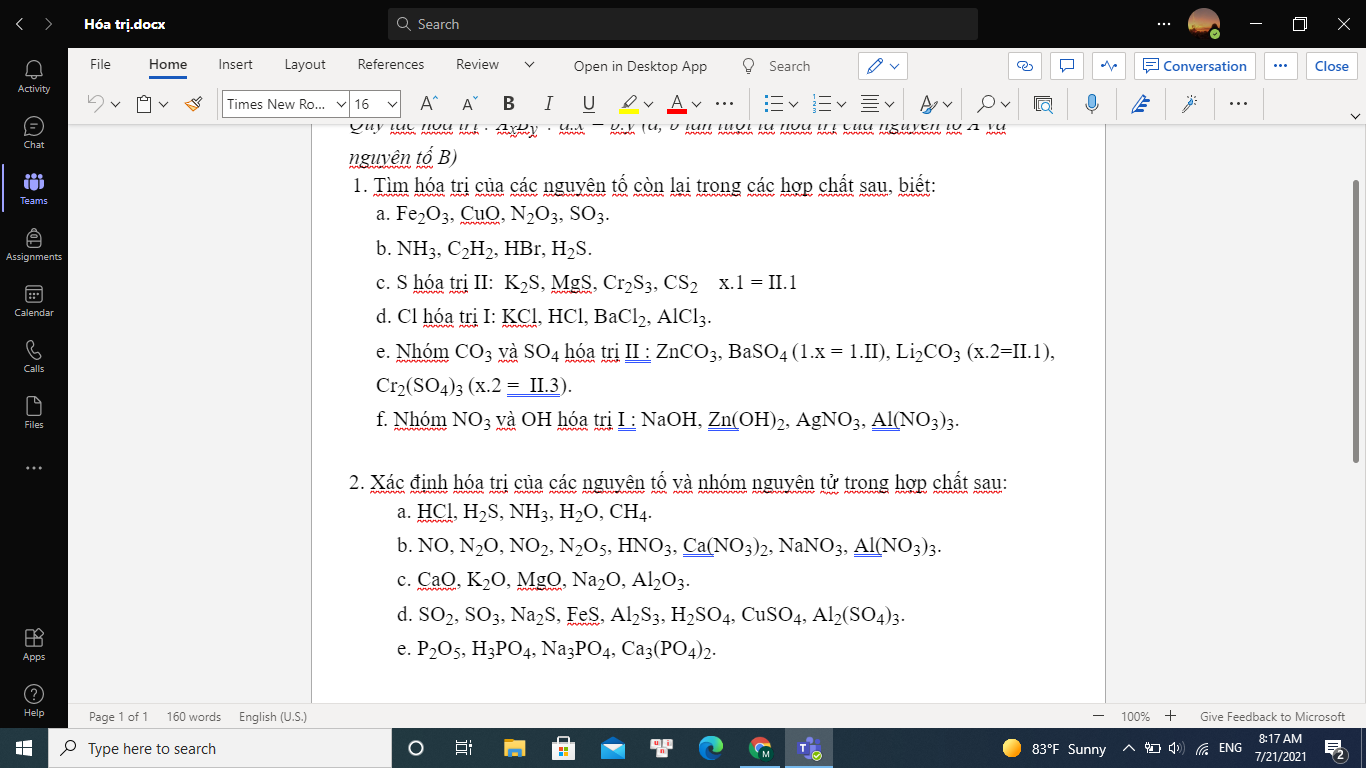
a) Fe2O3 : x.2=II.3 => x=III
CuO : x.1=II.1 => x=II
N2O3 : x.2=II.3 => x=III
SO3: x.1=II.3 => x= VI
b) NH3 : x.1=I.3 => x=III
C2H2 : C hóa trị IV, H hóa trị I ( do trong hợp chất hữu cơ, hóa trị của C luôn là IV)
HBr : I.1=x.1 => x=I
H2S: I.2=x.1 => x=II
c)K2S: x.2=II.1 => x=I
MgS : x.1=II.1 => x=II
Cr2S3 : x.2=II.3 => x=III
CS2: x.1=II.2=> x=IV
d) KCl: x.1=I.1=> x=I
HCl: x.1= 1.I => x=I
BaCl2 : x.1=I.2 => x=II
AlCl3 : x.1=I.3 => x=III
e) ZnCO3 : x.2=II.1 => x=II
BaSO4 : x.1=II.1 => x=II
Li2CO3 : x.2=II.1 => x=I
Cr2(SO4)3 : x.2=II.3 => x=III
f) NaOH : x.1=1.I => x=I
Zn(OH)2 : x.1=I.2 => x=II
AgNO3 : x.1=I.1 => x=I
Al(NO3)3 : x.1=I.3 => x=III
2.a) HCl : H(I), Cl(I)
H2S: H(I), S(II)
NH3 : N(III), H(I)
H2O : H(I), O(II)
CH4: C(IV), H(I)
b) NO: N(II), O(II)
N2O: N(I), O(II)
NO2: N(IV), O(II)
N2O5: N(V), O(II)
HNO3 : H(I), NO3 (I)
Ca(NO3)2 : Ca(II), NO3 (I)
NaNO3: Na(I), NO3 (I)
Al(NO3)3: Al (III),NO3 (I)
c) CaO: Ca(II), O(II)
K2O: K(I), O(II)
MgO : Mg(II), O(II)
Na2O: Na(I), O(II)
Al2O3: Al(III), O(II)
d) SO2: S(IV) ,O(II)
SO3: S(VI), O(II)
Na2S: Na(I), S(II)
FeS: Fe(II), S(II)
Al2S3: Al(III), S(II)
H2SO4: H(I), SO4(II)
CuSO4: Cu(II), SO4(II)
Al2(SO4)3: Al(III), SO4(II)
e) P2O5: P(V), O(II)
H3PO4: H(I), PO4(III)
Na3PO4: Na(I), PO4(III)
Ca3(PO4)2: Ca(II), PO4(III)

Đặt x là hóa trị kim loại M (x: nguyên dương)
nH2= 1,344/22,4=0,06(mol)
PTHH: M2Ox + x H2 -to-> 2 M + x H2O
Ta có: nM2Ox= 0,06/x(mol)
=>M(M2Ox)= 3,48 : (0,06/x)= 58x (g/mol)
Mặt khác: M(M2Ox)= 2.M(M)+16x
Không có TH thỏa

a)
$CH_3COOH + KOH \to CH_3COOK + H_2O$
n CH3COOH = n KOH = 0,1(mol)
$CH_3COOH + 2O_2 \xrightarrow{t^o} 2CO_2 + 2H_2O$
$C_2H_5OH + 3O_2 \xrightarrow{t^o} 2CO_2 + 3H_2O$
$CO_2 +C a(OH)_2 \to CaCO_3 + H_2O$
2n C2H5OH + 2n CH3COOH = n CO2 = n CaCO3 = 60/100 = 0,6(mol)
=> n C2H5OH = (0,6 - 0,1.2)/2 = 0,2(mol)
=> n O2 = 2n CH3COOH + 3n C2H5OH = 0,1.2 + 0,2.3 = 0,8(mol)
=> V O2 = 0,8.22,4 = 17,92 lít
b) m = 0,1.60 + 0,2.46 = 15,2 gam
%m CH3COOH = 0,1.60/15,2 .100% = 39,47%
%m C2H5OH = 100%- 39,47% = 60,53%









a,\(m_{HCl}=200.10\%=20\left(g\right)\Rightarrow n_{HCl}=\dfrac{20}{36,5}=0,548\left(mol\right)\)
b,\(n_{H_2SO_4}=0,2.0,2=0,04\left(mol\right)\)