Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{CO_2}=\dfrac{0.56}{22.4}=0.025\left(mol\right)\)
\(n_{KOH}=0.1\cdot0.6=0.06\left(mol\right)\)
\(2KOH+CO_2\rightarrow K_2CO_3+H_2O\)
\(0.05..........0.025.......0.025\)
\(m_{K_2CO_3}=0.025\cdot138=3.45\left(g\right)\)
\(C_{M_{KOH\left(dư\right)}}=\dfrac{0.01}{0.1}=0.1\left(M\right)\)
\(C_{M_{K_2CO_3}}=\dfrac{0.025}{0.1}=0.25\left(M\right)\)

n NaOH = 2 n CO 2 = 1,12x2 /22,4 = 0,1 (mol)
Nồng độ mol của dung dịch NaOH là 1M.

\(n_{CO_2}=\dfrac{2,8}{22,4}=0,125\left(mol\right)\)
PTHH :
\(CO_2+2KOH\rightarrow K_2CO_3+H_2O\)
0,125 0,25 0,125
\(a,C_{M\left(KOH\right)}=\dfrac{0,25}{0,1}=2,5\left(M\right)\)
\(b,C_{M\left(K_2CO_3\right)}=\dfrac{0,125}{0,1}=1,25\left(M\right)\)

\(n_{CO2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
a) Pt : \(CO_2+2KOH\rightarrow K_2CO_3+H_2O\)
\(n_{KOH}=2n_{CO2}=2.0,05=0,1\left(mol\right)\Rightarrow C_{MddKOH}=\dfrac{0,1}{0,1}=1M\)

1.
a, \(n_{CO_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
PTHH: CO2 + 2NaOH → Na2CO3 + H2O
Mol: 0,05 0,1
b, \(C_{M_{ddNaOH}}=\dfrac{0,1}{0,1}=1M\)
2.
a, \(m_{HCl}=200.7,3\%=14,6\left(g\right)\Rightarrow n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH: CuO + 2HCl → CuCl2 + H2O
Mol: 0,2 0,4 0,2
b,\(m_{CuO}=0,2.80=16\left(g\right)\)
c, \(C\%_{ddCuCl_2}=\dfrac{0,2.135.100\%}{16+200}=12,5\%\)

a) \(CO_2+2NaOH\rightarrow Na_2CO_3+H_2O\)
b) \(n_{CO_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
Theo PT: \(n_{NaOH}=2n_{CO_2}=0,1\left(mol\right)\)
=> \(CM_{NaOH}=\dfrac{0,1}{0,1}=1M\)
c) Sửa đề DNaOH = 1,2g/ml
\(m_{ddsaupu}=0,05.44+100.1,2=122,2\left(g\right)\)
\(n_{Na_2CO_3}=n_{CO_2}=0,05\left(mol\right)\)
=> \(C\%_{Na_2CO_3}=\dfrac{0,05.106}{122,2}.100=4,34\%\)

\(Pt: 2Al+3H_2SO_4 \rightarrow Al_2(SO_4)_3 + 3H_2\)
\(a.n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo pt: \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Al}=a=0,2.27=5,4\left(g\right)\)
\(b.n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{3}n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2g\)
\(c.\)Theo pt: \(n_{H_2SO_4}=n_{H_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,3.98=29,4g\)
\(C_{\%}H_2SO_4=\dfrac{29,4}{100}.100\%=29,4\%\)
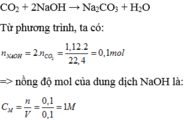
PTHH: \(CO_2+2NaOH\rightarrow Na_2CO_3+H_2O\)
Ta có: \(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Na_2CO_3}=0,3\left(mol\right)\\n_{NaOH}=0,6\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C\%_{NaOH}=\dfrac{0,6\cdot40}{240}\cdot100\%=10\%\\C\%_{Na_2CO_3}=\dfrac{0,3\cdot106}{240+0,3\cdot44}\cdot100\%\approx12,56\%\end{matrix}\right.\)
cám ơn nhìu ạ