Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đáp án D
nNaOH = 0,2.1,5=0,3 (mol)
NaOH + HCl → NaCl + H2O
0,3 → 0,3 (mol)
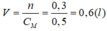

1)
- TN1:
\(n_{AgCl}=\dfrac{35,875}{143,5}=0,25\left(mol\right)\)
PTHH: AgNO3 + HCl --> AgCl + HNO3
0,25<--0,25
TN2:
nNaOH = 0,5.0,3 = 0,15 (mol)
PTHH: NaOH + HCl --> NaCl + H2O
0,15--->0,15
\(n_{HCl\left(dd.C\right)}=0,25+0,15\) = 0,4 (mol)
=> \(C_{M\left(dd.C\right)}=\dfrac{0,4}{2}=0,2M\)
2)
Có \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{V}M\\C_{M\left(B\right)}=\dfrac{0,15}{V^,}M\end{matrix}\right.\)
nHCl(A) = \(\dfrac{0,025}{V}\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
\(\dfrac{0,025}{V}\)------>\(\dfrac{0,0125}{V}\)
nHCl(B) = \(\dfrac{0,015}{V^,}\) (mol)
PTHH: Fe + 2HCl --> FeCl2 + H2
\(\dfrac{0,015}{V^,}\)-------->\(\dfrac{0,0075}{V^,}\)
TH1: \(\dfrac{0,0125}{V}=\dfrac{0,0075}{V^,}+0,02\)
Mà V + V' = 2 (l)
=> \(\left[{}\begin{matrix}V=1,5;V^,=0,5\left(KTM\right)\\V=0,5;V^,=1,5\left(TM\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{0,5}=0,5M\\C_{M\left(B\right)}=\dfrac{0,15}{1,5}=0,1M\end{matrix}\right.\)
TH2: \(\dfrac{0,0125}{V}+0,02=\dfrac{0,0075}{V^,}\)
=> \(\left[{}\begin{matrix}V=\dfrac{1+\sqrt{6}}{2};V^,=\dfrac{3-\sqrt{6}}{2}\\V=\dfrac{1-\sqrt{6}}{2}\left(L\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{\dfrac{1+\sqrt{6}}{2}}=\dfrac{-1+\sqrt{6}}{10}M\\C_{M\left(B\right)}=\dfrac{0,15}{\dfrac{3-\sqrt{6}}{2}}=\dfrac{3+\sqrt{6}}{10}M\end{matrix}\right.\)

$200ml=0,2l$
$n_{NaOH}=0,2.1,5=0,3(mol)$
$H^++OH^-\to H_2O$
$\to n_{H^+}=n_{HCl}=n_{OH^-}=n_{NaOH}=0,3(mol)$
$\to V_{dd\,HCl}=\dfrac{0,3}{0,4}=0,75(l)=750(ml)$

Ta có nH+ cần dùng sẽ gấp đôi số mol khí (dùng bảo toàn e)
=> n HCl = 0,12 mol
=> V = 120 ml

nH2=0,6mol
PTHH: 2Al+6HCl=>2AlCl3+3H2
0,4<-1,2<--0,4 <- 0,6
=> mAl=0,4.27=10,8g
=> m AL2O3=21-10,8=10,2g
=> nAl2O3=0,1mol
PTHH: Al2O3+6HCl=> 2AlCl3+3H2O
0,1--->0,6------>0,2----->0,3
PTHH: AlCl3+3NaOH=> Al(OH)3+3NaCl
nAl(OH)3=0,4mol
nAlCl3=0,4+0,2=0,6mol
ta có : 0,6 : 0,4
=> n AlCl3 dư theo n nAl(OH)3
p/ư: 0,4<-1,2<------0,4--->1,2
=> V (NaOH) cần dùng là : V=1,2:0,5=2,4l

\(Cl_2+2NaBr\rightarrow2NaCl+Br_2\\Cl_2+2NaI\rightarrow2NaCl+I_2\\ n_{NaBr}=1.0,2=0,2\left(mol\right)\\ n_{NaI}=0,2.2=0,4\left(mol\right)\\ n_{Cl_2}=\dfrac{n_{NaBr}+n_{NaI}}{2}=\dfrac{0,2+0,4}{2}=0,3\left(mol\right)\\ V_{Cl_2\left(đktc\right)}=0,3.22,4=6,72\left(lít\right)\)

a, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: M + 2H2O → M(OH)2 + H2
Mol: 0,1 0,1 0,1
\(\Rightarrow M_M=\dfrac{4}{0,1}=40\left(g/mol\right)\)
⇒ M là canxi (Ca)
\(C\%_{ddCa\left(OH\right)_2}=\dfrac{0,1.74.100\%}{500}=1,48\%\)
b) \(m_{Ca\left(OH\right)_2}=200.1,48=2,96\left(g\right)\Rightarrow n_{Ca\left(OH\right)_2}=\dfrac{2,96}{74}=0,04\left(mol\right)\)
PTHH: Ca(OH)2 + 2HCl → CaCl2 + 2H2O
Mol: 0,04 0,08
\(V_{ddHCl}=\dfrac{0,08}{2}=0,04\left(l\right)=40\left(ml\right)\)
Đáp án D