Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,Gọi\left\{{}\begin{matrix}n_{CH_4}=a\left(mol\right)\\n_{C_2H_4}=b\left(mol\right)\\n_{C_2H_2}=c\left(mol\right)\end{matrix}\right.\\ n_{hhkhí}=0,4\left(mol\right)\\ n_{CO_2}=\dfrac{15,68}{22,4}=0,7\left(mol\right)\\ n_{Br_2}=\dfrac{64}{160}=0,4\left(mol\right)\\ PTHH:C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ Mol:a\rightarrow a\\ C_2H_2+2Br_2\rightarrow C_2H_2Br_4\\ Mol:b\rightarrow2b\\ CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\\ Mol:a\rightarrow2a\rightarrow a\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\\ Mol:b\rightarrow3b\rightarrow2b\\ 2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\\ Mol:c\rightarrow2,5c\rightarrow2c\\ Hệ.pt\left\{{}\begin{matrix}a+b+c=0,4\\b+2c=0,4\\a+2b+2c=0,7\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,2\left(mol\right)\\c=0,1\left(mol\right)\end{matrix}\right.\)
\(\%V_{CH_4}=\%V_{C_2H_2}=\dfrac{0,1}{0,4}=25\%\\ \%V_{C_2H_4}=\dfrac{0,2}{0,4}=50\%\)
\(m_{CH_4}=0,1.16=1,6\left(g\right)\\ m_{C_2H_4}=28.0,2=5,6\left(g\right)\\ m_{C_2H_2}=0,1.26=2,6\left(g\right)\\ \%m_{CH_4}=\dfrac{1,6}{1,6+5,6+2,6}=16,32\%\\ \%m_{C_2H_4}=\dfrac{5,6}{1,6+5,6+2,6}=57,14\%\\ \%m_{C_2H_2}=100\%-16,32\%-57,14\%=26,54\%\)
\(b,PTHH:C_2H_5OH\rightarrow C_2H_4+H_2O\\ Mol:0,2\leftarrow0,2\\ m_{C_2H_5OH}=0,2.46=9,2\left(g\right)\)
Dài quá!!!

\(a,n_{hhkhí\left(C_2H_4,C_2H_2\right)}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{Br_2}=\dfrac{80}{160}=0,5\left(mol\right)\\ Gọi\left\{{}\begin{matrix}n_{C_2H_4}=a\left(mol\right)\\n_{C_2H_2}=b\left(mol\right)\end{matrix}\right.\\ PTHH:C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ Mol:a\rightarrow a\\ C_2H_2+2Br_2\rightarrow C_2H_2Br_4\\ Mol:b\rightarrow2b\\ Hệ.pt\left\{{}\begin{matrix}a+b=0,3\\a+2b=0,5\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,2\left(mol\right)\end{matrix}\right.\\ \%V_{C_2H_4}=\dfrac{0,1}{0,3}=33,33\%\\ \%V_{C_2H_2}=100\%-33,335=66,67\%\)
\(b,PTHH:\\ C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\\ Mol:0,1\rightarrow0,3\rightarrow0,2\\ 2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\\ Mol:0,2\rightarrow0,25\rightarrow0,4\\ n_{CO_2}=0,2+0,4=0,6\left(mol\right)\\ PTHH:Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\\ Mol:0,6\rightarrow0,6\rightarrow0,6\\ m_{CaCO_3}=0,6.100=60\left(g\right)\)

mtăng = mC2H4 = 2,8 (g)
=> \(n_{C_2H_4}=\dfrac{2,8}{28}=0,1\left(mol\right)\)
=> VC2H4 = 0,1.22,4 = 2,24 (l)
=> VCH4 = 4,48 - 2,24 = 2,24 (l)

\(n_{hhkhí}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\\ m_{tăng}=m_{C_2H_4}=4,2\left(g\right)\\ n_{C_2H_4}=\dfrac{4,2}{28}=0,15\left(mol\right)\\ n_{CH_4}=0,35-0,15=0,2\left(mol\right)\\ \left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,15}{0,35}=42,85\%\\\%V_{CH_4}=100\%-42,85\%=57,15\%\end{matrix}\right.\)
PTHH:
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,15 ------------------> 0,3
CH4 + O2 --to--> CO2 + 2H2O
0,2 -----------------> 0,2
Ca(OH)2 + CO2 ---> CaCO3 + H2O
0,5 -------> 0,5
\(m_{CaCO_3}=0,5.100=50\left(g\right)\)

a) mtăng = mC2H4
=> \(n_{C_2H_4}=\dfrac{5,6}{28}=0,2\left(mol\right)\)
=> \(\%V_{C_2H_4}=\dfrac{0,2.22,4}{13,44}.100\%=33,33\%\)
\(\%V_{CH_4}=100\%-33,33\%=66,67\%\)
b) \(n_{CH_4}=\dfrac{13,44.66,67\%}{22,4}=0,4\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,4--------------->0,4
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,2----------------->0,4
Ca(OH)2 + CO2 --> CaCO3 + H2O
0,8----->0,8
=> mCaCO3 = 0,8.100 = 80 (g)
a.\(m_{tăng}=m_{C_2H_4}=5,6g\)
\(n_{hh}=\dfrac{13,44}{22,4}=0,6mol\)
\(n_{C_2H_4}=\dfrac{5,6}{28}=0,2mol\)
\(\%V_{C_2H_4}=\dfrac{0,2}{0,6}.100=33,33\%\)
\(\%V_{CH_4}=100\%-33,33\%=66,67\%\)
b.\(C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\)
0,2 0,4 ( mol )
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
0,4 0,4 ( mol )
\(n_{CO_2}=0,4+0,4=0,8mol\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
0,8 0,8 ( mol )
\(m_{CaCO_3}=0,8.100=80g\)

a. Phương trình phản ứng :
C2H2 + 2Br2 → C2H2Br4 (1)
C2H4 + Br2 → C2H4Br2 (2)
b. Hỗn hợp khí B gồm có H2, C2H6. Gọi x, y ( mol ) lần lượt là số mol của H2 và C2H6 có trong 6,72 lít hỗn hợp B.
nB = x + y = 6,72 : 22,4 = 0,3 mol (I)
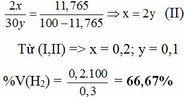
% V(C2H6) = 100% – 66,67% = 33,33%
c. nA = 11,2 : 22,4 = 0,5 mol , M A = 0,4 . 44 = 17,6 g/ mol
mA = 0,5 . 17,6 = 8,8 gam
mB = 0,2 . 2 + 0,1 . 30 = 3,4 gam
Vậy khối lượng bình Br2 tăng: m = mA – mB = 8,8 – 3,4 = 5,4 gam.

\(a,n_{Br_2}=\dfrac{32}{160}=0,2\left(mol\right)\\ PTHH:C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ Theo.pt:n_{C_2H_4}=n_{Br_2}=0,2\left(mol\right)\\ n_{hhkhi}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{CO_2}=0,3-0,2=0,1\left(mol\right)\\ m_{C_2H_4}=0,2.28=5,6\left(g\right)\\ m_{CO_2}=0,1.44=4,4\left(g\right)\\ b,C_{MddBr_2}=\dfrac{0,2}{0,5}=0,4M\)

a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(FeO+2HCl\rightarrow FeCl_2+H_2O\)
b, Ta có: \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Al}=0,2.27=5,4\left(g\right)\)
⇒ mFeO = 12,6 - 5,4 = 7,2 (g)
c, Phần này đề cho dd NaOH dư hay vừa đủ bạn nhỉ?
d, Cho hh vào dd H2SO4 đặc nguội thì có khí thoát ra.
PT: \(2FeO+4H_2SO_{4\left(đ\right)}\rightarrow Fe_2\left(SO_4\right)_3+SO_2+4H_2O\)
Ta có: \(n_{FeO}=\dfrac{7,2}{72}=0,1\left(mol\right)\)
Theo PT: \(n_{SO_2}=\dfrac{1}{2}n_{FeO}=0,05\left(mol\right)\)
\(\Rightarrow V_{SO_2}=0,05.22,4=1,12\left(l\right)\)

a)
$V_{CH_4} = V_{khí\ thoát\ ra} = 2,24(lít)$
$\%V_{CH_4} = \dfrac{2,24}{8,96}.100\% = 25\%$
$\%V_{C_2H_4} = 100\% -25\% = 75\%$
b)
$n_{Br_2} = n_{C_2H_4} = \dfrac{8,96.75\%}{22,4} = 0,3(mol)$
$C_{M_{Br_2}} = \dfrac{0,3}{0,2} = 1,5M$
$m_{tăng} = m_{C_2H_4} = 0,3.28 = 8,4(gam)$