Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Al}=\dfrac{13,5}{27}=0,5\left(mol\right)\\a, 4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ n_{O_2}=\dfrac{3}{4}.0,5=0,375\left(mol\right)\\ m_{O_2}=32.0,375=12\left(g\right)\\ V_{O_2\left(đktc\right)}=0,375.22,4=8,4\left(l\right)\)

nAl = \(\dfrac{5,4}{27}=0,2\) mol
nO2 = \(\dfrac{6,4}{32}=0,2\) mol
Pt: 4Al + 3O2 --to--> 2Al2O3
0,2 mol->0,15 mol-> 0,1 mol
Xét tỉ lệ mol giữa Al và O2:
\(\dfrac{0,2}{4}< \dfrac{0,2}{3}\)
Vậy O2 dư
mAl2O3 = 0,1 . 102 = 10,2 (g)
mO2 dư = (0,2 - 0,15) . 32 = 1,6 (g)
4Al + 3O2 + 2Al2O3
0,2 0,15 0,1
nAl = m/M=5,4/27=0,2
mAl2O3= n.M=0,1×(27×2+16×3)=10,2g

\(n_{Al}=\dfrac{8,1}{27}=0,3(mol);n_{HCl}=\dfrac{21,9}{36,5}=0,6(mol)\\ a,PTHH:2Al+6HCl\to 2AlCl_3+3H_2\\ LTL:\dfrac{0,3}{2}>\dfrac{0,6}{6}\Rightarrow Al\text { dư}\\ n_{Al(dư)}=0,3-\dfrac{0,6}{3}=0,1(mol)\\ \Rightarrow m_{Al(dư)}=0,1.27=2,7(g)\\ c,n_{AlCl_3}=\dfrac{1}{3}n_{HCl}=0,2(mol)\\n_{H_2}=\dfrac{1}{2}n_{HCl}=0,3(mol)\\ \Rightarrow m_{AlCl_3}=0,2.133,5=26,7(g)\\ d,V_{H_2}=0,3.22,4=6,72(l)\)

Đáp án B

Khối lượng oxi sau phản ứng là m = 0,05.32 = 1,6 g

PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Ta có: \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{H_2SO_4}=0,15\left(mol\right)=n_{H_2}\\n_{Al_2\left(SO_4\right)_3}=0,05\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{H_2SO_4}=0,15\cdot98=14,7\left(g\right)\\m_{Al_2\left(SO_4\right)_3}=0,05\cdot342=17,1\left(g\right)\\V_{H_2}=0,15\cdot22,4=3,36\left(l\right)\end{matrix}\right.\)
a) \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
______0,1--->0,15-------->0,05------->0,15
=> mH2SO4 = 0,15.98 = 14,7 (g)
b) VH2 = 0,15.22,4 = 3,36 (l)
c) mAl2(SO4)3 = 0,05.342 = 17,1 (g)

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ n_{HCl}=\dfrac{25,55}{36,5}=0,7\left(mol\right)\\a. 2Al+6HCl\rightarrow2AlCl_3+3H_2\\ b.Vì:\dfrac{0,2}{2}< \dfrac{0,7}{6}\\ \Rightarrow HCldư\\ n_{HCl\left(dư\right)}=0,7-\dfrac{6}{2}.0,2=0,1\left(mol\right)\\ n_{AlCl_3}=n_{Al}=0,2\left(mol\right)\\ n_{H_2}=\dfrac{3}{2}.0,2=0,3\left(mol\right)\\ m_{H_2}=0,3.2=0,6\left(g\right)\\ m_{HCl\left(dư\right)}=0,1.36,5=3,65\left(g\right)\\ m_{AlCl_3}=133,5.0,2=26,7\left(g\right)\)
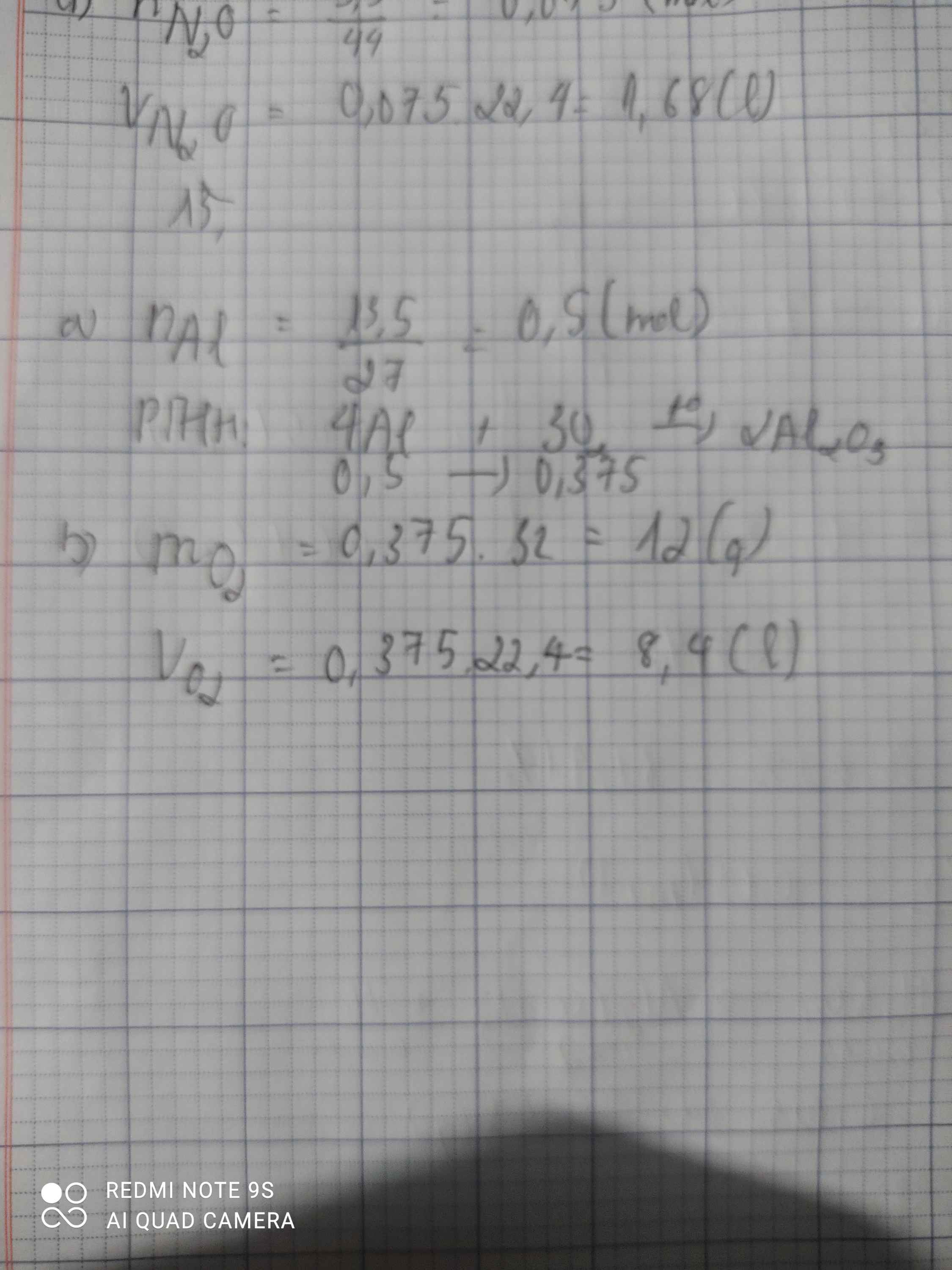
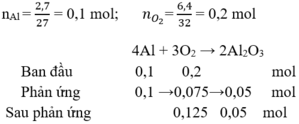
\(n_{O_2}=\dfrac{3,2}{32}=0,1\left(mol\right)\)
\(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
PTHH :
\(4Al+3O_2\rightarrow2Al_2O_3\)
2/15 0,1 1/15
\(\dfrac{0,1}{3}< \dfrac{0,1}{2}\)
---> Tính theo O2
\(m_{Al}=\dfrac{2}{15}.27=3,6\left(g\right)\)