Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi nFe = a (mol); nMg = b (mol)
56a + 24b = 8 (1)
nH2 = 4,48/22,4 = 0,2 (mol)
PTHH:
Fe + 2HCl -> FeCl2 + H2
a ---> a ---> a ---> a
Mg + 2HCl -> MgCl2 + H2
b ---> b ---> b ---> b
a + b = 0,2 (2)
(1)(2) => a = b = 0,1 (mol)
mFe = 0,1 . 56 = 5,6 (g)
%mFe = 5,6/8 = 70%
%mMg = 100% - 70% = 30%
nHCl = 0,1 . 2 + 0,1 . 2 = 0,4 (mol)
CMddHCl = 0,4/0,1 = 4M

Gọi số mol Al, Fe là a, b (mol)
=> 27a + 56b = 11,1 (1)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
a----------------------->1,5a
Fe + 2HCl --> FeCl2 + H2
b------------------------>b
=> 1,5a + b = 0,3 (2)
(1)(2) => a = 0,1; b = 0,15
=> \(\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{11,1}.100\%=24,32\%\\\%m_{Fe}=\dfrac{0,15.56}{11,1}.100\%=75,68\end{matrix}\right.\)

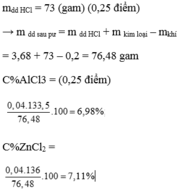
\(2Al+6HCl->2AlCl_3+3H_2\\ Fe+2HCl->FeCl_2+H_2\\ n_{Al}=a;n_{Fe}=b\\ 27a+56b=8,3\\ 1,5a+b=\dfrac{5,6}{22,4}=0,25\\ a=b=0,1\\ m_{Al}=27\cdot0,1=2,7g\\ m_{Fe}=8,3-2,7=5,6g\\ a=\dfrac{3a+2b}{500}\cdot36,5=3,65\%\\ m_{ddsau}=508,3-0,25\cdot2=507,8g\\ C\%_{AlCl_3}=\dfrac{133,5a}{507,8}=2,63\%\\ C\%_{FeCl_2}=\dfrac{127b}{507,8}=2,50\%\)

a)
Gọi $n_{Zn} = a(mol) ; n_{Al} = b(mol) \Rightarrow 65a + 27b = 11,9(1)$
$Zn + 2HCl \to ZnCl_2 + H_2$
$2Al + 6HCl \to 2AlCl_3 + 3H_2$
Theo PTHH :
$n_{H_2} = a + 1,5b = \dfrac{8,96}{22,4} = 0,4(2)$
Từ (1)(2) suy ra : a = 0,1; b = 0,2
$m_{Zn} = 0,1.65 = 6,5(gam)$
$m_{Al} = 0,2.27 = 5,4(gam)$
b) $n_{HCl} = 2n_{H_2} = 0,8(mol)$
$C\%_{HCl} = \dfrac{0,8.36,5}{125}.100\% = 23,36\%$

Fe+2HCl->FeCl2+H2
x---2x-----------x
Mg+2HCl->MgCl2+H2
y------2y-----------y
Ta có :
\(\left\{{}\begin{matrix}56x+24y=24\\x+y=\dfrac{13,44}{22,4}\end{matrix}\right.\)
=>x=0,3 mol, y=0,3 mol
=>%m Fe=\(\dfrac{0,3.56}{24}.100\)=70%
=>%m Mg=100-70=30%
=>VHCl=\(\dfrac{0,3.2+0,3.2}{2}\)=0,6l=600ml
b)
XCl2+2AgNO3->2AgCl+X(NO3)2
0,6--------------------1,2mol
=>m AgCl=1,2.143,5=172,2g

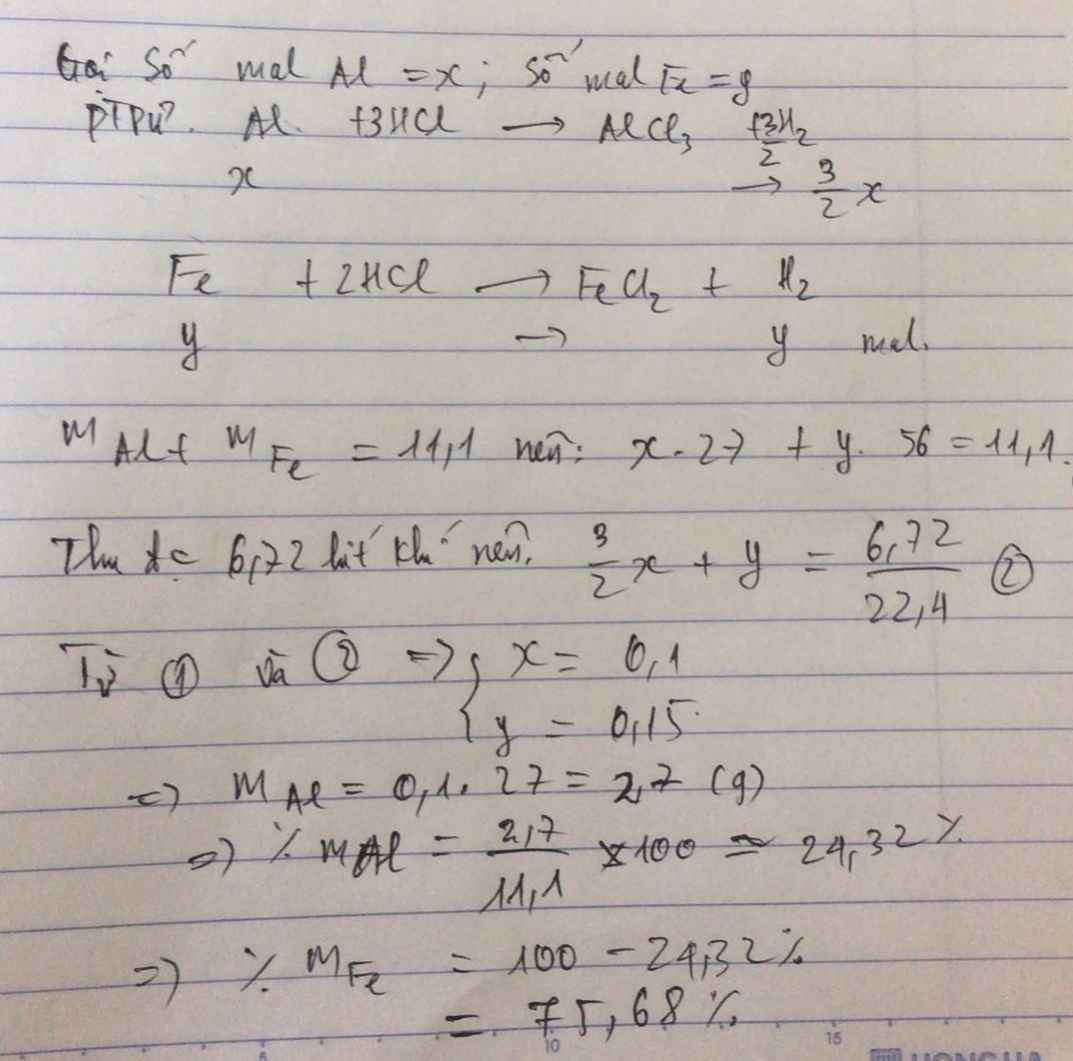

\(a.BTNT\left(H\right):n_{HCl}=2n_{H_2}=0,65\left(mol\right)\\ \Rightarrow CM_{HCl}=\dfrac{0,65}{0,5}=1,3M\\ b.2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Đặt:\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}\dfrac{3}{2}x+y=0,325\\27x+56y=9,65\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{Al}=4,05\left(g\right)\\m_{Fe}=5,6\left(g\right)\end{matrix}\right.\)