Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Ta có: \(n_{NO}+n_{NO_2}+n_{N_2}=\dfrac{22,4}{22,4}=1\left(mol\right)\left(1\right)\)
Mà: mX = 35,8 (g)
\(\Rightarrow30n_{NO}+46n_{NO_2}+28n_{N_2}=35,8\left(2\right)\)
Có: \(n_{Al}=\dfrac{32,4}{27}=1,2\left(mol\right)\)
\(n_{Cu}=\dfrac{22,4}{64}=0,35\left(mol\right)\)
BT e, có: 3nNO + nNO2 + 10nN2 = 3nAl + 2nCu = 4,3 (3)
Từ (1), (2) và (3) \(\Rightarrow\left\{{}\begin{matrix}n_{NO}=0,3\left(mol\right)\\n_{NO_2}=0,4\left(mol\right)\\n_{N_2}=0,3\left(mol\right)\end{matrix}\right.\)
⇒ nHNO3 = 4nNO + 2nNO2 + 12nN2 = 5,6 (mol)

a) Gọi \(n_{Mg}=4x\left(mol\right)\Rightarrow n_{Al}=5x\left(mol\right)\)
=> \(24.4x+27.5x=6,93\Leftrightarrow x=0,03mol\)
=> \(n_{Mg}=4.0,03=0,12mol\Rightarrow m_{Mg}=2,88g,mAl=6,93-2,88=4,05g\)
b) pt:
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
0,12 0,24
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,15 0,45
=> nHCl = 0,24+0,45=0,69 mol
=> VHCl = 0,69:4=0,1725 lít

Đáp án C.
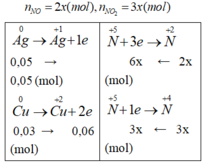
9x = 0,11; x= 11/900 => V = 5x.22,4 = 1,368 (l)

a) \(\left\{{}\begin{matrix}24.n_{Mg}+27.n_{Al}=6,93\\\dfrac{n_{Mg}}{n_{Al}}=\dfrac{4}{5}\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{Mg}=0,12\left(mol\right)\\n_{Al}=0,15\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}m_{Mg}=0,12.24=2,88\left(g\right)\\m_{Al}=0,15.27=4,05\left(g\right)\end{matrix}\right.\)
b)
PTHH: Mg + 2HCl --> MgCl2 + H2
0,12->0,24
2Al + 6HCl --> 2AlCl3 + 3H2
0,15-->0,45
=> nHCl(min) = 0,24 + 0,45 =0,69 (mol)
=> \(V_{dd.HCl\left(min\right)}=\dfrac{0,69}{4}=0,1725\left(l\right)\)

Gọi nZn = x , nCu = y (mol)
=> 65x+64y = 12,9 (1)
nkhí = \(\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
dk/H2 = 19 => Mkhí = 38
NO 30 8 1
38
NO2 46 8 1
=> \(\dfrac{nNO}{nNO_2}=1:1\)
=> nNO = 0,1(mol) , nNO2 = 0,1 (mol)
Zn => Zn+2 + 2e x 2x | N+5 + 3e => N+2 0,3 0,1 |
Cu => Cu+2 + 2e y 2y | N+5 + 1e => N+4 0,1 0,1 |
=> 2x + 2y = 0,4 (mol) (2)
Từ 1 + 2 => x = 0,1, y = 0,1 (mol)
=> mCu = 6,4g
mZn = 6,5g

Bài 1:
a+b) PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)=n_{Fe}\)
\(\Rightarrow m_{Fe}=0,2\cdot56=11,2\left(g\right)\) \(\Rightarrow m_{Cu}=6,4\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{11,2}{17,6}\cdot100\%\approx63,64\%\\\%m_{Cu}=36,36\%\end{matrix}\right.\)
c) Ta có: \(n_{Cu}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
Bảo toàn nguyên tố: \(n_{Fe_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Fe}=0,1\left(mol\right)=n_{CuSO_4}\)
\(\Rightarrow m_{muối}=0,1\cdot400+0,1\cdot160=56\left(g\right)\)
Bài 2:
Quy đổi hh gồm Fe (a mol) và O (b mol)
\(\Rightarrow56a+16b=27,6\) (1)
Ta có: \(n_{SO_2}=\dfrac{5,04}{22,4}=0,225\left(mol\right)\)
Bảo toàn electron: \(3n_{Fe}=2n_O+2n_{SO_2}\) \(\Rightarrow3a-2b=0,45\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,39\\b=0,36\end{matrix}\right.\)
Bảo toàn nguyên tố: \(n_{Fe_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Fe}=0,195\left(mol\right)\) \(\Rightarrow m_{Fe_2\left(SO_4\right)_3}=0,195\cdot400=78\left(g\right)\)
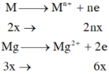
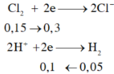
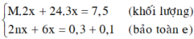
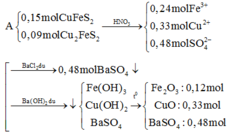

a)\(\left\{{}\begin{matrix}n_{Cu}=x\left(mol\right)\\n_{Al}=y\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}64x+27y=9,1\\BTe:2x+3y=0,5\cdot1\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,1\end{matrix}\right.\)
\(\%m_{Cu}=\dfrac{0,1\cdot64}{9,1}\cdot100\%=70,33\%\)
\(\%m_{Al}=100-70,33\%=29,67\%\)
b)\(\left\{{}\begin{matrix}n_{NO_2}+n_{NO}=0,5\\\dfrac{n_{NO_2}}{n_{NO}}=\dfrac{2}{1}\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}n_{NO_2}=\dfrac{1}{3}\\n_{NO}=\dfrac{1}{6}\end{matrix}\right.\)
Gọi \(\left\{{}\begin{matrix}n_{Cu}=a\left(mol\right)\\n_{Al}=b\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}64a+27b=9,1\\BTe:2x+3y=\dfrac{1}{3}\cdot1+\dfrac{1}{6}\cdot3\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}a=\dfrac{4}{115}\\b=\dfrac{527}{2070}\end{matrix}\right.\)
\(\%m_{Cu}=\dfrac{\dfrac{4}{115}\cdot64}{9,1}\cdot100\%=24,46\%\)
\(\%m_{Al}=100\%-24,46\%=75,54\%\)
\(n_{HNO_3}=2n_{NO_2}+4n_{NO}=2\cdot\dfrac{1}{3}+4\cdot\dfrac{1}{6}=\dfrac{4}{3}mol\)