Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

$C_2H_4 + Br_2 \to C_2H_4Br_2$
Ta có :
$m_{C_2H_4} = m_{dd\ tăng} = 3(gam)$
$\Rightarrow n_{C_2H_4} = \dfrac{3}{28}(mol)$
$\Rightarrow n_{C_2H_6} = 0,25 - \dfrac{3}{28} = \dfrac{1}{7}(mol)$
$\%V_{C_2H_4} = \dfrac{ \dfrac{3}{28} }{0,25}.100\% = 42,9\%4
$\%V_{C_2H_6} = 100\% - 42,9\% = 57,1\%$
$\%m_{C_2H_4} = \dfrac{3}{3 + \dfrac{1}{7}.30}.100\% = 41,2\%$
$\%m_{C_2H_6} = 100\% - 41,2\% = 58,8\%$
$C_2H_4 + Br_2 \to C_2H_4Br_2$
Ta có :
$m_{C_2H_4} = m_{dd\ tăng} = 3(gam)$
$\Rightarrow n_{C_2H_4} = \dfrac{3}{28}(mol)$
$\Rightarrow n_{C_2H_6} = 0,25 - \dfrac{3}{28} = \dfrac{1}{7}(mol)$
$\%V_{C_2H_4} = \dfrac{ \dfrac{3}{28} }{0,25}.100\% = 42,9\%$
$\%V_{C_2H_6} = 100\% - 42,9\% = 57,1\%$
$\%m_{C_2H_4} = \dfrac{3}{3 + \dfrac{1}{7}.30}.100\% = 41,2\%$
$\%m_{C_2H_6} = 100\% - 41,2\% = 58,8\%$

a) \(V_{CH_4}=0,6\left(l\right)\)
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,6}{1,2}.100\%=50\%\\\%V_{C_2H_4}=100\%-50\%=50\%\end{matrix}\right.\)
b) \(n_{C_2H_4}=\dfrac{1,2-0,6}{24}=0,025\left(mol\right)\)
PTHH: C2H4 + Br2 --> C2H4Br2
0,025-->0,025
=> \(m_{Br_2}=0,025.160=4\left(g\right)\)
c)
\(n_{CH_4}=\dfrac{0,6}{24}=0,025\left(mol\right)\)
=> nH = 0,025.4 = 0,1 (mol)
\(n_{Cl_2}=\dfrac{0,72}{24}=0,03\left(mol\right)\)
=> nCl(thế H) = 0,03 (mol)
Do nH > nCl(thế H)
=> H không bị thế hoàn toàn bởi Cl
=> nHCl = 0,03 (mol)
=> mHCl = 0,03.36,5 = 1,095 (g)

PTHH: \(K_2O+2HCl\rightarrow2KCl+H_2O\)
\(K_2SO_3+2HCl\rightarrow2KCl+SO_2\uparrow+H_2O\)
a) Ta có: \(\left\{{}\begin{matrix}n_{HCl}=\dfrac{200\cdot14,6\%}{36,5}=0,8\left(mol\right)\\n_{SO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}n_{K_2SO_3}=0,3\left(mol\right)\\n_{K_2O}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{K_2O}=\dfrac{0,1\cdot94}{0,1\cdot94+0,3\cdot158}\cdot100\%\approx16,55\%\\\%m_{K_2SO_3}=83,45\%\end{matrix}\right.\)
b) Theo các PTHH: \(n_{KCl}=0,8\left(mol\right)\) \(\Rightarrow m_{KCl}=74,5\cdot0,8=59,6\left(g\right)\)
Mặt khác: \(\left\{{}\begin{matrix}m_{hh}=56,8\left(g\right)\\m_{SO_2}=0,3\cdot64=19,2\left(g\right)\end{matrix}\right.\)
\(\Rightarrow m_{dd}=m_{hh}+m_{ddHCl}-m_{SO_2}=237,6\left(g\right)\)
\(\Rightarrow C\%_{KCl}=\dfrac{59,6}{237,6}\cdot100\%\approx25,1\%\)

a) \(C_2H_4 + Br_2 \to C_2H_4Br_2\)
b)
\(n_{C_2H_4} = n_{Br_2} = \dfrac{5,6}{160} = 0,035(mol)\\ \%V_{C_2H_4} = \dfrac{0,035.22,4}{0,86}.100\% = 91,16\%\\ \%V_{CH_4} = 100\% - 91,16\% = 8,84\%\)
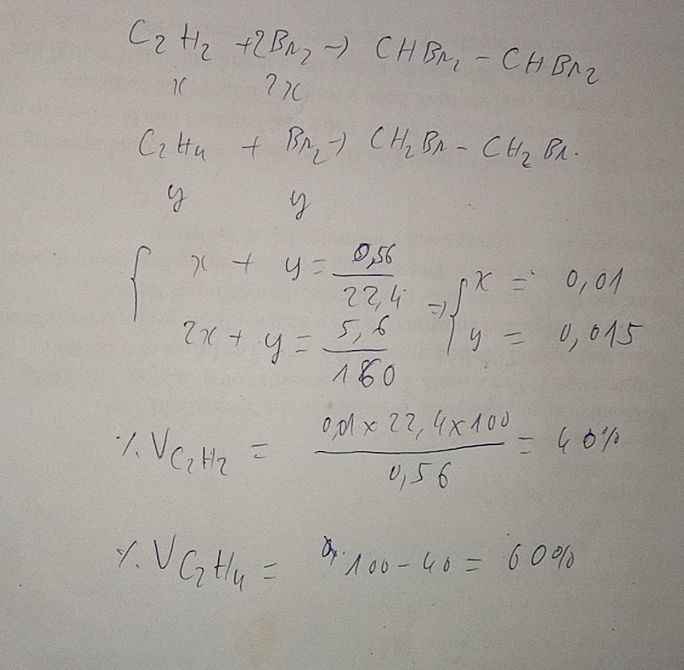
\(n_{hh}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(m_{Br_2}=200\cdot\dfrac{20}{100}=40\left(g\right)\)
\(n_{Br_2}=\dfrac{40}{160}=0.25\left(mol\right)\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
\(0.25........0.25..........0.25\)
\(\)\(n_{C_2H_4}=n_{hh}=0.25\left(mol\right)\)
=> Sai đề