Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1:
\(a,2Cu+O_2\underrightarrow{t^o}2CuO\)
b, \(n_{O_2}=\dfrac{1,12}{32}=0,035mol\)
\(n_{Cu}=\dfrac{6,4}{64}=0,1mol\)
\(\dfrac{0,1}{2}>\dfrac{0,035}{1}\) => Cu dư, O2 đủ
\(n_{Cu}\left(dư\right)=0,1-0,07=0,039\left(mol\right)\)
c, \(m_{CuO}=0,07.80=5,6g\)
Bài 2:
\(n_{Al}=\dfrac{13,5}{27}=0,5mol\)
\(n_{O_2}=\dfrac{6,67}{32}=0,21\left(mol\right)\)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(\dfrac{0,5}{4}>\dfrac{0,21}{3}\) => Al dư, O2 đủ
\(n_{Al_2O_3}=\dfrac{2}{3}.0,21=0,14\left(mol\right)\)
\(m_{Al_2O_3}=0,14.102=14,28g\)

a, PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,15.22,4=3,36\left(l\right)\)
c, Theo PT: \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)

a: \(4Al+3O_2\rightarrow2Al_2O_3\)
b: \(n_{Al}=\dfrac{21.6}{27}=0.8\left(mol\right)\)
\(\Leftrightarrow n_{Al_2O_3}=0.4\left(mol\right)\)
\(m_{Al_2O_3}=0.4\cdot102=40.8\left(g\right)\)
c: \(n_{O_2}=0.6\left(mol\right)\)
\(V_{O_2}=0.6\cdot22.4=13.44\left(lít\right)\)
a) 4Al + 3O2 --to--> 2Al2O3
b) \(n_{Al}=\dfrac{21,6}{27}=0,8\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,8-->0,6-------->0,4
=> \(m_{Al_2O_3}=0,4.102=40,8\left(g\right)\)
c) \(V_{O_2}=0,6.22,4=13,44\left(l\right)\)
d) \(V_{kk}=13,44:20\%=67,2\left(l\right)\)

nAl=\(\dfrac{5,4}{27}\)=0,2mol
nO2=\(\dfrac{4,48}{22,4}\)=0,2mol
PTHH:
4Al + 3O2--to->2Al2O3
Tỉ lệ \(\dfrac{0,2}{4}\) <\(\dfrac{0,2}{3}\)->Al hết O2 dưtính theo Al
=>m O2=\(\dfrac{1}{60}\).32=\(\dfrac{8}{15}\)g
2KMnO4-to>K2MnO4+MnO2+O2
0,4--------------------------------------0,2
m KMnO4=0,4.158=63,2g
.

Bài 1 :
a. \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
\(n_{O_2}=\dfrac{16}{32}=0,5\left(mol\right)\)
b. PTHH : 4Al + 3O2 -to> 2Al2O3
0,4 0,3 0,2
Xét tỉ lệ : \(\dfrac{0,4}{4}< \dfrac{0,5}{3}\) => Al đủ , O2 dư
\(m_{O_2\left(dư\right)}=\left(0,5-0,3\right).32=6,4\left(g\right)\)
c. \(m_{Al_2O_3}=0,2.102=20,4\left(g\right)\)
Bài 2:
| Các thời điểm | Fe2O3 (gam) | CO (lít) | Fe(gam) | CO2(lít) | dkhí/H2 |
| Thời điểm t0 | 16 | 8,96 | 11,2 | 6,72 | 20 |
| Thời điểm t1 | 3,2 | 1,344 | 2,24 | 1,344 | 22 |
| Thời điểm t2 | 128/15 | 3,584 | 448/75 | 3,584 | 22 |
| Thời điểm t3 | 16 | 6,72 | 11,2 | 6,72 | 22 |

\(n_{Al}=\dfrac{m}{M}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ n_{O_2}=\dfrac{m}{M}=\dfrac{12,8}{32}=0,4\left(mol\right)\)
\(PTHH:4Al+3O_2-^{t^o}>2Al_2O_3\)
tỉ lệ: 4 : 3 : 2
n(mol) 0,2 0,4
m(mol p/u) 0,2-->0,15---->0,1
\(\dfrac{n_{Al}}{4}< \dfrac{n_{O_2}}{3}\left(\dfrac{0,2}{4}< \dfrac{0,4}{3}\right)\)
`=>` `Al` hết , `O_2` dư
`=>` tính theo `Al`
\(n_{O_2\left(dư\right)}=0,4-0,15=0,25\left(mol\right)\\ m_{O_2\left(dư\right)}=n\cdot M=0,25\cdot32=8\left(g\right)\\ m_{Al_2O_3}=n\cdot M=0,1\cdot\left(27\cdot2+16\cdot3\right)=10,2\left(g\right)\)

nFe = 2.8/56 = 0.05 (mol)
nO2 = 22.4 / 22.4 = 1 (mol)
3Fe + 2O2 -to-> Fe3O4
0.05__1/30______1/60
mO2 (dư) = ( 1 - 1/30) * 32 = 30.93 (g)
mFe3O4 = 1/60 * 232 = 3.867 (g)
a/ \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
b/ Ta có: \(n_{Fe}=\dfrac{2.8}{56}=0.05\left(mol\right)\)
\(n_{O_2}=\dfrac{22.4}{22.4}=1\left(mol\right)\)
Ta có: \(\dfrac{n_{Fe\left(bra\right)}}{n_{Fe\left(pt\right)}}=\dfrac{0.05}{3}=0.016< \dfrac{n_{O_2\left(bra\right)}}{n_{O_2\left(pt\right)}}=\dfrac{1}{2}=0.5\)
=> Oxi phản ứng dư
mO2 dư = (1 - 1/30) . 32 = 30.93 (g)
mFe3O4 = 1/60 . 232 = 3.867 (g)
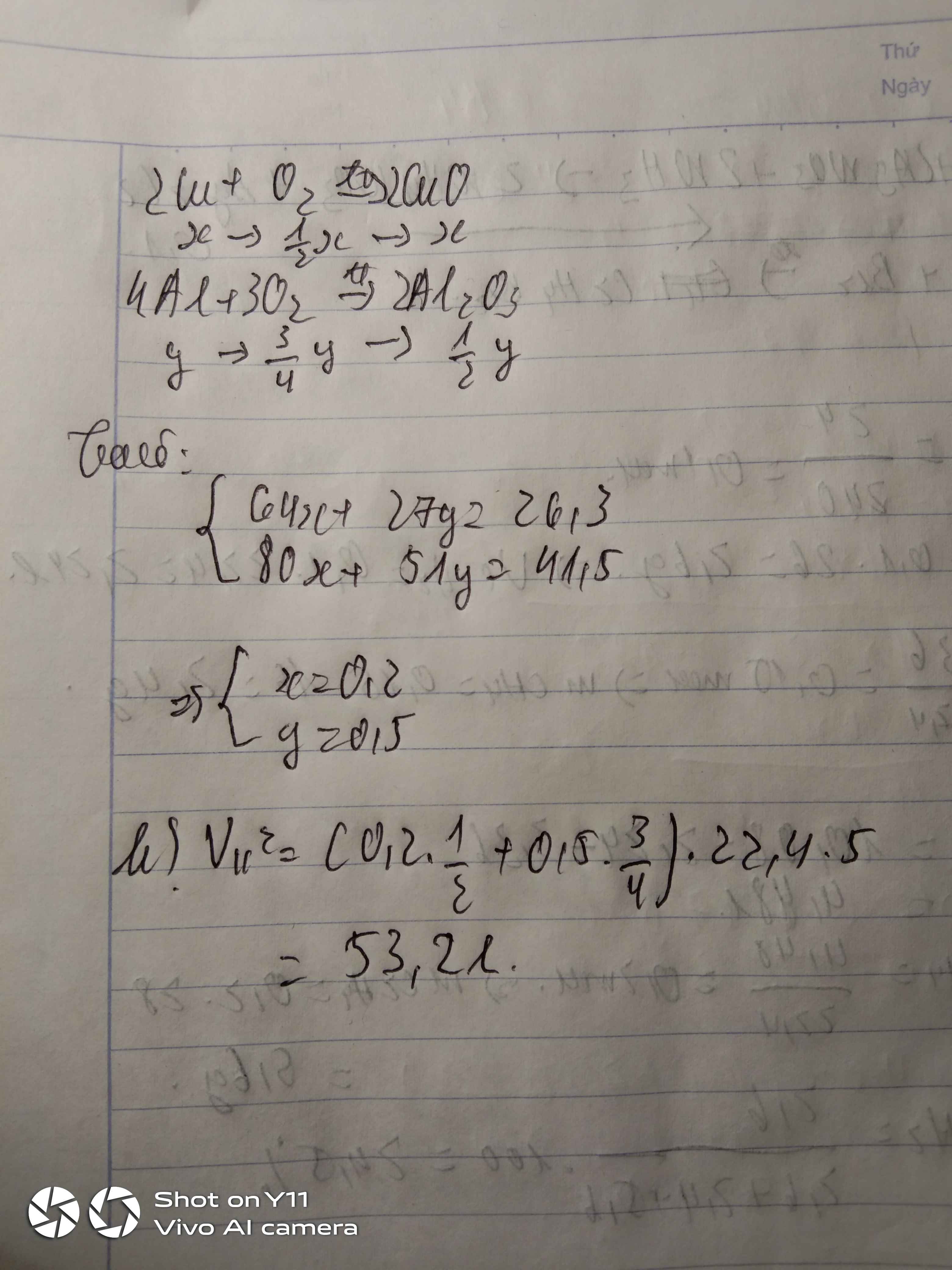
\(n_{Al}=\dfrac{m}{M}=\dfrac{10,8}{27}=0,4\left(mol\right)\\ a.PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
4 3 2
0,4 0,3 0,2
\(m_{Al_2O_3}=n.M=0,2.\left(27.2+16.3\right)=20,4\left(g\right)\\ c.V_{O_2}=n.24,79=0,3.24,79=7,437\left(l\right)\)
\(d.n_{O_2}=\dfrac{m}{M}=\dfrac{12,8}{\left(16.2\right)}=0,4\left(mol\right)\\ PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
4 3 2
0,53 0,4 0,27
Tỉ lệ: \(\dfrac{0,53}{4}< \dfrac{0,4}{3}< \dfrac{0,27}{2}\Rightarrow Al_2O_3\) dư và dư \(m_{Al_2O_3}=n.M=0,27.\left(27.2+16.3\right)=27,54\left(g\right).\)
a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
Theo PT: \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,2\left(mol\right)\Rightarrow m_{Al_2O_3}=0,2.102=20,4\left(g\right)\)
c, \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,3\left(mol\right)\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
d, \(n_{O_2}=\dfrac{12,8}{32}=0,4\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,4}{4}< \dfrac{0,4}{3}\), ta được O2 dư.
\(\Rightarrow n_{O_2\left(dư\right)}=0,4-0,3=0,1\left(mol\right)\Rightarrow m_{O_2\left(dư\right)}=0,1.32=3,2\left(g\right)\)