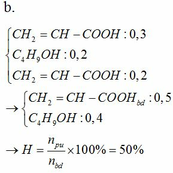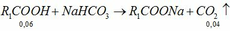Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\) (1)
\(2CH_3COOH+2Na\rightarrow2CH_3COONa+H_2\) (2)
a) Ta có: \(n_{H_2}=\frac{4,48}{22,4}=0,2\left(mol\right)\)
Đặt số mol của \(C_2H_5OH\) là \(a\) \(\Rightarrow n_{H_2\left(1\right)}=\frac{1}{2}a\)
Đặt số mol của \(CH_3COOH\) là \(b\) \(\Rightarrow n_{H_2\left(2\right)}=\frac{1}{2}b\)
Ta có hệ phương trình:
\(\left\{{}\begin{matrix}46a+60b=20,5\\\frac{1}{2}a+\frac{1}{2}b=0,2\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,25\\b=0,15\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}n_{C_2H_5OH}=0,25mol\\n_{CH_3COOH}=0,15mol\end{matrix}\right.\)
\(\Rightarrow m_{CH_3COOH}=60\cdot0,15=9\left(g\right)\)
\(\Rightarrow\%m_{CH_3COOH}=\frac{9}{20,5}\cdot100\approx43,9\%\)
\(\Rightarrow\%m_{C_2H_5OH}=56,1\%\)
b) PTHH: \(C_2H_5OH+CH_3COOH\underrightarrow{xt}CH_3COOC_2H_5+H_2O\)
Xét tỷ lệ: \(\frac{0,15}{1}< \frac{0,25}{1}\) \(\Rightarrow\) Axit phản ứng hết, Rượu còn dư
\(\Rightarrow n_{CH_3COOC_2H_5}=0,15mol\) \(\Rightarrow m_{este}=0,15\cdot88=13,2\left(g\right)\)
\(\Rightarrow m_{este}thực=13,2\cdot90\%=11,88\left(g\right)\)

nC2H5OH = 8.05/46 = 0.175 (mol)
nCH3COOH = 36/60 = 0.6 (mol)
nCH3COOC2H5 = 12.32/88 = 0.14 (mol)
C2H5OH + CH3COOH <-H2SO4đ,t0-> CH3COOC2H5 + H2O
1.......................1
0.175................0.6
LTL : 0.175/1 < 0.6/1
=> CH3COOH dư
mCH3COOH (dư) = ( 0.6 - 0.175) * 60 = 25.5 (g)
nCH3COOC2H5 = nC2H5OH = 0.175 (mol)
H% = 0.14/0.175 * 100% = 80%

Gọi \(\left\{{}\begin{matrix}n_{ancol}:x\left(mol\right)\\n_{axit}:y\left(mol\right)\end{matrix}\right.\)
PTHH:
\(C_2H_5OH+K\rightarrow C_2H_5ONa+\frac{1}{2}H_2\)
x_____________________________0,5x
\(CH_3COOH+K\rightarrow CH_3COONa+\frac{1}{2}H_2\)
y____________________________________0,5y
Giải hệ PT:
\(\left\{{}\begin{matrix}46x+60y=20,5\\0,5x+0,5y=\frac{4,48}{22,4}=0,2\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,25\\y=0,15\end{matrix}\right.\)
\(PTHH:C_2H_5OH+CH_3COOH⇌CH_3COOC_2H_5+H_2O\)
\(\Rightarrow n_{CH3COOC2H5}=n_{CH3COOH}=0,15\left(mol\right)\)
\(\Rightarrow m_{este}=13,2\left(g\right)\)

1,
- Xét phần 2:
\(n_{CH_3COOC_2H_5}=\dfrac{4,4}{88}=0,05\left(mol\right)\)
PTHH: CH3COOH + C2H5OH \(\xrightarrow[t^o]{H_2SO_{4\left(đ\right)}}\) CH3COOC2H5 + H2O
LTL: 0,5a < 0,5b (do a < b) => C2H5OH dư
Theo pthh: \(n_{CH_3COOH\left(pư\right)}=n_{CH_3COOC_2H_5}=0,05\left(mol\right)\)
Mà H = 50%
=> \(n_{CH_3COOH\left(bđ\right)}=\dfrac{0,05}{50\%}=0,1\left(mol\right)\)
Xét phần 2:
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PTHH:
2CH3COOH + 2Na ---> 2CH3COONa + H2
0,1--------------------------------------------->0,05
2C2H5OH + 2Na ---> 2C2H5ONa + H2
0,4<----------------------------------------0,2
=> Trong X có: \(\left\{{}\begin{matrix}n_{CH_3COOH}=0,1.2=0,2\left(mol\right)\\n_{C_2H_5OH}=0,4.2=0,8\left(mol\right)\end{matrix}\right.\)
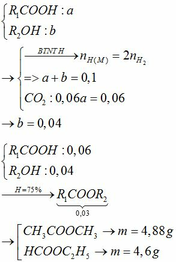
2, Gọi \(\left\{{}\begin{matrix}n_{CH_3COOH}=a\left(mol\right)\\n_{C_2H_5OH}=b\left(mol\right)\end{matrix}\right.\left(a,b>0\right)\)
\(n_{H_2O}=\dfrac{23,4}{18}=1,3\left(mol\right)\)
PTHH:
CH3COOH + 2O2 --to--> 2CO2 + 2H2O
a------------------------------------------>2a
C2H5OH + 3O2 --to--> 2CO2 + 3H2O
b-------------------------------------->3b
=> Hệ pt \(\left\{{}\begin{matrix}60a+46b=25,8\\2a+3b=1,3\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\left(mol\right)\\b=0,3\left(mol\right)\end{matrix}\right.\left(TM\right)\)
PTHH: \(CH_3COOH+C_2H_5OH\xrightarrow[t^o]{H_2SO_{4\left(đ\right)}}CH_3COOC_2H_5+H_2O\)
LTL: 0,2 < 0,3 => Rượu dư
Theo pthh: \(n_{CH_3COOH\left(pư\right)}=n_{CH_3COOC_2H_5}=\dfrac{14,08}{88}=0,16\left(mol\right)\)
=> \(H=\dfrac{0,16}{0,2}.100\%=80\%\)