Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
\(C\%_{dd_{HCl}}=\dfrac{m_{HCl}}{mdd_{HCl}}\cdot100\%=\dfrac{20}{600}\cdot100\%=3,33\%\)
b)
\(mdd_{NaCl}=m_{NaCl}+m_{H_2O}=15+45=60\left(g\right)\)
=> \(C\%dd_{NaCl}=\dfrac{m_{NaCl}}{mdd_{NaCl}}\cdot100\%=\dfrac{15}{60}\cdot100\%=25\%\)
hok tốt nhé

\(C\)\(\%\)\(=\dfrac{m_{ct}}{m_{dd}} .100\)\(\%\)= \(\dfrac{20}{500} . 100\)\(\%\)\(=4 \)\(\%\)

\(a,C\%_{NaCl}=\dfrac{15}{15+185}.100\%=7,5\%\\ b,m_{HNO_3}=\dfrac{18,9}{100}.100+\dfrac{6,3}{100}.200=31,5\left(g\right)\\ m_{ddHNO_3}=100+200=300\left(g\right)\\ C\%_{HNO_3}=\dfrac{31,5}{300}.100\%=10,5\%\)
\(c,n_{NaCl}=\dfrac{5,85}{58,5}=0,1\left(mol\right)\\ C_{M\left(NaCl\right)}=\dfrac{0,1}{0,1}=1M\\ d,n_{KOH}=2.0,2+0,2.0,2=0,44\left(mol\right)\\ V_{ddKOH}=0,2+0,2=0,4\left(l\right)\\ C_{M\left(KOH\right)}=\dfrac{0,44}{0,4}=1,1M\\ e,m_{NaOH}=\dfrac{150.16}{100}=24\left(g\right)\\ m_{ddNaOH}=50+150=200\left(g\right)\\ C\%_{NaOH}=\dfrac{24}{200}.100\%=12\%\)



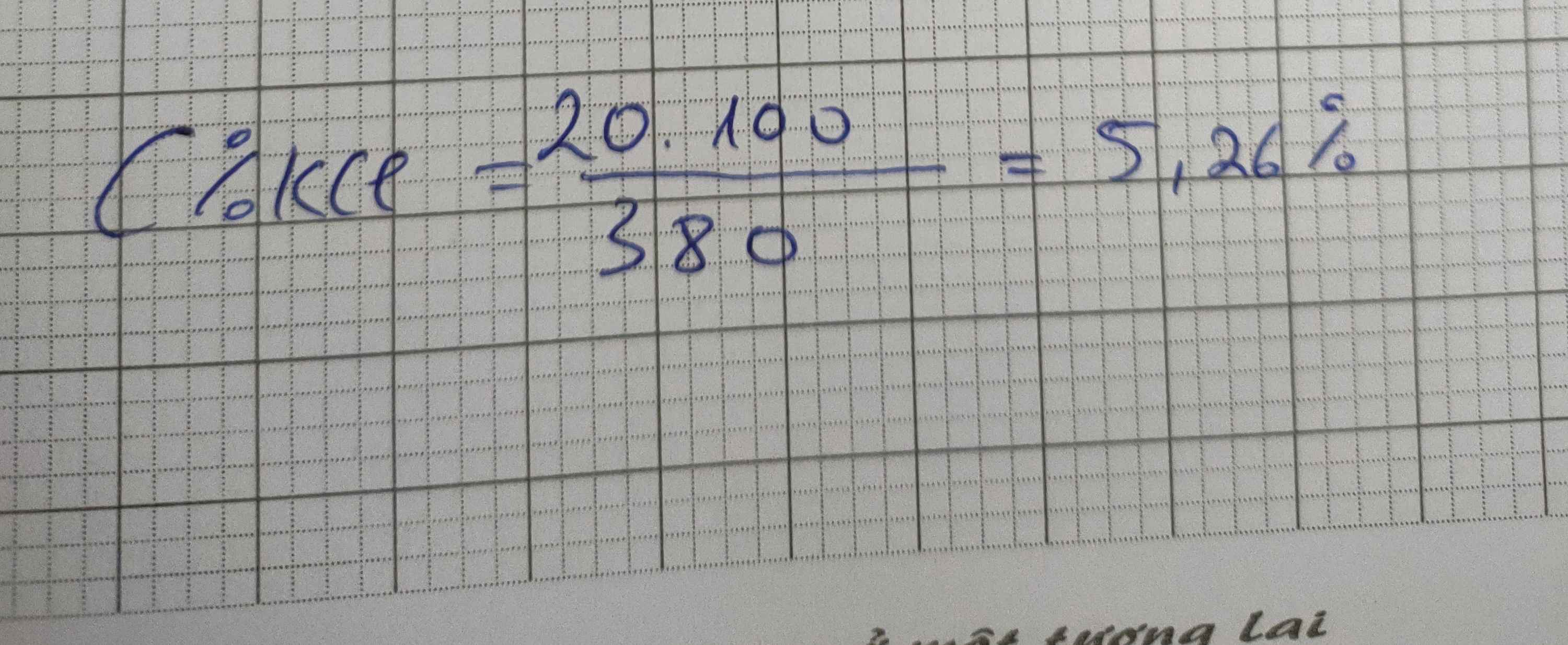
\(C\%_{KCl}=\dfrac{20}{600}\cdot100\%=3.33\%\)
\(C\%_{K_2SO_4}=\dfrac{75}{1500}\cdot100\%=5\%\)
\(C\%_{NaCl}=\dfrac{15}{15+45}\cdot100\%=25\%\)
\(n_{HCl}=\dfrac{4.48}{22.4}=0.2\left(mol\right)\)
\(m_{HCl}=0.2\cdot36.5=7.3\left(g\right)\)
\(m_{dd_{HCl}}=7.3+500=507.3\left(g\right)\)
\(C\%_{HCl}=\dfrac{7.3}{507.3}\cdot100\%=1.44\%\)