Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a.\(n_{KMnO_4}=\dfrac{m_{KMnO_4}}{M_{KMnO_4}}=\dfrac{3,16}{158}=0,02mol\)
\(2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\)
0,02 0,01 ( mol )
\(V_{O_2}=n_{O_2}.22,4=0,01.22,4=0,224l\)
b.
\(4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\)
1/75 0,01 1/150 ( mol )
\(m_{Al}=n_{Al}.M_{Al}=\dfrac{1}{75}.27=0,36g\)
\(m_{Al_2O_3}=n_{Al_2O_3}.M_{Al_2O_3}=\dfrac{1}{150}.102=0,68g\)
2KMnO4-to>K2MnO4+MnO2+O2
0,02-------------------------------------0,01
4Al+3O2-to->2Al2O3
\(\dfrac{1}{75}\)---0,01---------\(\dfrac{1}{150}\)
n KMnO4=\(\dfrac{3,16}{158}\)=0,02 mol
=>VO2=0,01.22,4=0,224 l
b)m Al=\(\dfrac{1}{75}\).27=0,36g
=>m Al2O3=\(\dfrac{1}{150}\)102=0,68g

Câu 1)
a) 2HgO\(-t^0\rightarrow2Hg+O_2\)
b)Theo gt: \(n_{HgO}=\frac{2,17}{96}\approx0,023\left(mol\right)\\ \)
theo PTHH : \(n_{O2}=\frac{1}{2}n_{HgO}=\frac{1}{2}\cdot0,023=0,0115\left(mol\right)\\ \Rightarrow m_{O2}=0,0115\cdot32=0,368\left(g\right)\)
c)theo gt:\(n_{HgO}=0,5\left(mol\right)\)
theo PTHH : \(n_{Hg}=n_{HgO}=0,5\left(mol\right)\\ \Rightarrow m_{Hg}=0,5\cdot80=40\left(g\right)\)
Câu 2)
a)PTHH : \(S+O_2-t^0\rightarrow SO_2\)
b)theo gt: \(n_{SO2}=\frac{2,24}{22,4}=0,1\left(mol\right)\)
theo PTHH \(n_S=n_{SO2}=0,1\left(mol\right)\\ \Rightarrow m_S=0,1\cdot32=3,2\left(g\right)\)
Ta có khối lượng S tham gia là 3,25 g , khối lượng S phản ứng là 3,2 g
Độ tinh khiết của mẫu lưu huỳnh là \(\frac{3,2}{3,25}\cdot100\%\approx98,4\%\)
c)the PTHH \(n_{O2}=n_{SO2}=0,1\left(mol\right)\Rightarrow m_{O2}=0,1\cdot32=3,2\left(g\right)\)

a) V O2 cần dùng= 20 . 100=2000 ml=2 (l)
--> n O2 =\(\frac{2}{22,4}\)=\(\frac{5}{56}\)(mol)
2KMnO4 --t*--> K2MnO4 + MnO2 + O2
\(\frac{5}{28}\) <------- \(\frac{5}{56}\)(mol)
m KMnO4 = \(\frac{5}{28}\). 158 . (100% + 10%)= 31,04 (g)
b) 2KClO3 ----t*,V2O5----> 2KCl + 3O2 (nhiệt độ, xúc tác)
\(\frac{5}{84}\) <------- \(\frac{5}{56}\)(mol)
m KClO3=\(\frac{5}{84}\).122,5= 7,29(g)
a) Thể tích oxi cần dùng là : (lít).
Số mol khí oxi là : = 0,099 (mol).
Phương trình phản ứng :
2KMnO4 K2MnO4 + MnO2 + O2
2mol 1mol
n mol 0,099 mol
=> n = = 0,198 (mol).
Khối lượng Kali pemaganat cần dùng là :
m = 0,198. (39 + 55 + 64) = 31,3 (g).
b) Phương trình hóa học.
KClO3 2KCl + 3O2
2.122,5 gam 3.22,4 lít
m gam 2,22 lít
Khối lượng kali clorat cần dùng là :
m = (gam).

a)PTHH:2KClO\(_3\)➞\(^{t^o}\)2KCl+3O\(_2\)
b) n\(_{KClO_3}\)=\(\dfrac{m_{KClO_3}}{M_{KClO_3}}\)=\(\dfrac{12,15}{122,5}\)\(\approx\)0,1(m)
PTHH : 2KClO\(_3\) ➞\(^{t^o}\) 2KCl + 3O\(_2\)
tỉ lệ : 2 2 3
số mol : 0,1 0,1 0,15
V\(_{O_2}\)=n\(_{O_2}\).22,4=0,15.22,4=3,36(l)
c)PTHH : 2Zn + O\(_2\) -> 2ZnO
tỉ lệ : 2 1 2
số mol :0,3 0,15 0,3
m\(_{Zn}\)=n\(_{Zn}\).M\(_{Zn}\)=0,3.65=19,5(g)

a, \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
b, Ta có: \(n_{Fe}=\dfrac{50,4}{56}=0,9\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{2}{3}n_{Fe}=0,6\left(mol\right)\Rightarrow V_{O_2}=0,6.22,4=13,44\left(l\right)\)
c, \(n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=0,3\left(mol\right)\Rightarrow m_{Fe_3O_4}=0,3.232=69,6\left(g\right)\)
d, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,4\left(mol\right)\Rightarrow m_{KClO_3}=0,4.122,5=49\left(g\right)\)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{50,4}{56}=0,9\left(mol\right)\)
\(a.PTHH:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
3 2 1
0,9 0,6 0,3
\(b.V_{O_2}=n.24,79=0,6.24,79=14,874\left(l\right)\)
\(c.m_{Fe_3O_4}=n.M=0,3.\left(56.3+16.4\right)=69,6\left(g\right)\)
\(d.V_{O_2}=14,874\left(l\right)\\ \Rightarrow n_{O_2}=\dfrac{V}{24,79}=\dfrac{14,874}{24,79}=0,6\left(mol\right)\\ PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
2 2 3
0,6 0,6 0,9
\(m_{KClO_3}=n.M=0,6.\left(39+35,5+16.3\right)=55,5\left(g\right).\)

\(a,PTHH:2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\\ b,n_{KMnO_4}=\dfrac{15,8}{158}=0,1\left(mol\right)\\ n_{O_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ V_{O_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ c,4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\\ n_{P_2O_5}=\dfrac{2}{5}.n_{O_2}=\dfrac{2}{5}.0,05=0,02\left(mol\right)\\ m_{P_2O_5}=0,02.142=2,84\left(g\right)\)

a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{Al_2O_3}=\dfrac{20,4}{102}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{2}n_{Al_2O_3}=0,3\left(mol\right)\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
c, \(V_{kk}=\dfrac{V_{O_2}}{20\%}=33,6\left(l\right)\)

a.\(n_{KMnO_4}=\dfrac{m}{M}=\dfrac{31,6}{158}=0,2mol\)
\(PTHH:2KMnO_4\underrightarrow{np}K_2MnO_4+MnO_2+O_2\)
2 1 1 1 ( mol )
0,2 0,1
\(V_{O_2}=n.22,4=0,1.22,4=2,24l\)
b.\(n_{Fe}=\dfrac{m}{M}=\dfrac{11,2}{56}=0,2mol\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
3 2 1 ( mol )
0,2 0,1
0,1 0,1 0,05 ( mol )
\(m_{Fe_3O_4}=n.M=0,05.232=11,6g\)
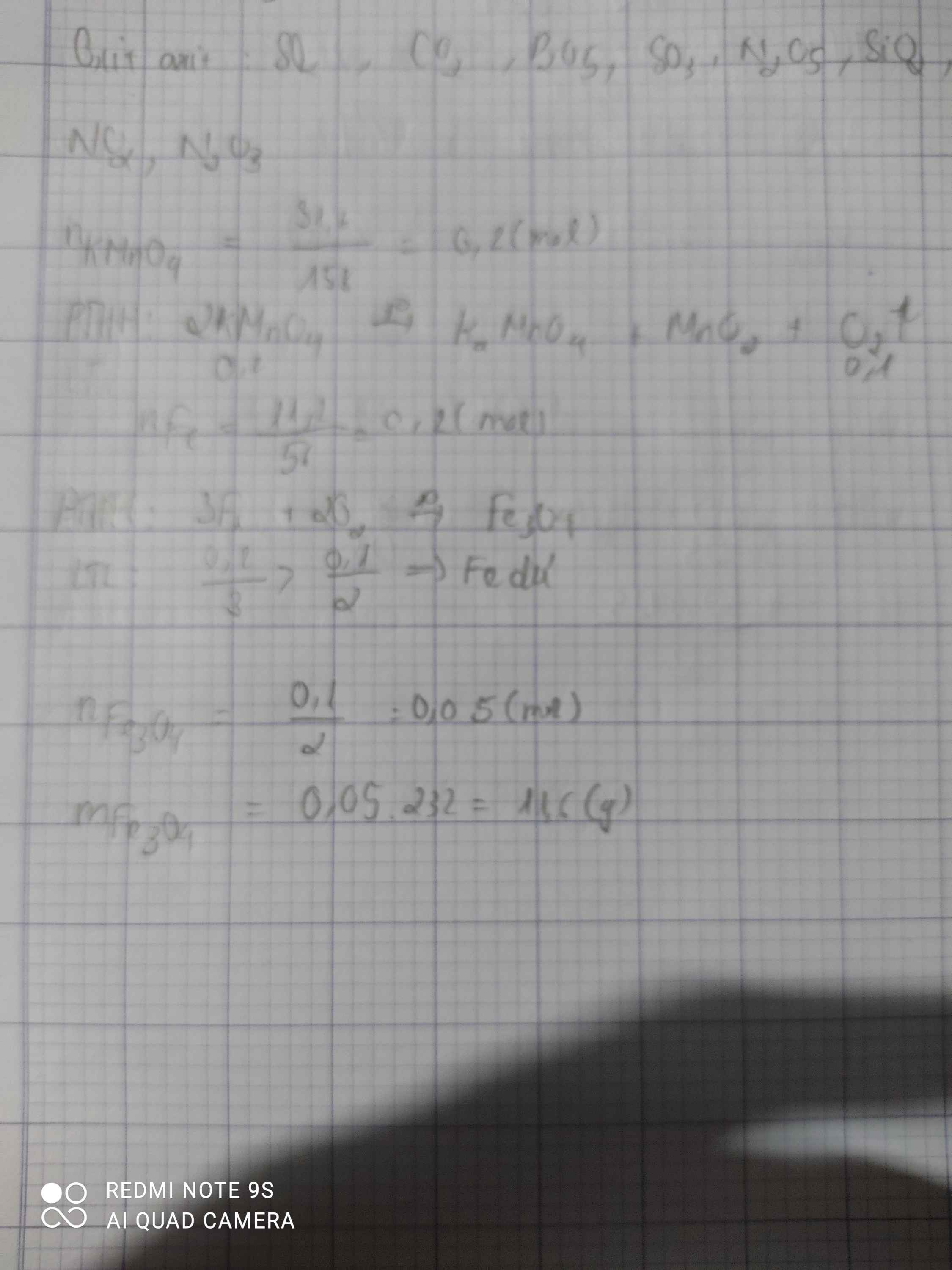
Câu 3.
a.b.\(n_{KClO_3}=\dfrac{24,5}{122,5}=0,2mol\)
\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,2 0,3 ( mol )
\(V_{O_2}=0,3.22,4=6,72l\)
c.\(n_{Al}=\dfrac{5,4}{27}=0,2mol\)
\(4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\)
0,2 < 0,3 ( mol )
0,2 0,1 ( mol )
\(m_{Al_2O_3}=0,1.102=10,2g\)
Câu 4.
a.b.
\(n_{KClO_3}=\dfrac{12,25}{122,5}=0,1mol\)
\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,1 0,15 ( mol )
\(V_{O_2}=0,15.22,4=3,36l\)
c.\(n_{Fe}=\dfrac{8,4}{56}=0,15mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,15 < 0,15 ( mol )
0,15 0,05 ( mol )
\(m_{Fe_3O_4}=0,05.232=11,6g\)