Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) $2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
b)
$n_{H_2SO_4} = \dfrac{200.15\%}{98} = \dfrac{15}{49}(mol)$
Theo PTHH :
$n_{H_2} = n_{H_2SO_4} = \dfrac{15}{49}(mol)$
$n_{Al_2(SO_4)_3} = \dfrac{1}{3}n_{H_2SO_4} = \dfrac{5}{49}(mol)$
Vậy :
$V_{H_2} = \dfrac{15}{49}.22,4 = 6,86(lít)$
$m_{Al_2(SO_4)_3} = \dfrac{5}{49}.342 = 34,9(gam)$
\(n_{H_2SO_4}=\dfrac{200\cdot15\%}{98}=\dfrac{15}{49}\left(mol\right)\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(........\dfrac{15}{49}.........\dfrac{5}{49}......\dfrac{15}{49}\)
\(m_{Al_2\left(SO_4\right)_3}=\dfrac{5}{49}\cdot342=35\left(g\right)\)
\(V_{H_2}=\dfrac{15}{49}\cdot22.4=6.85\left(l\right)\)

B1 : nFe = 11,2 /56 = 0,2 (mol)
Fe+ 2HCl -- . FeCl2 + H2
mFeCl2 = 0,2.127 = 25,4 (g)
VH2 = 0,2 .22,4 = 4,48 (l)
mHCl = 0,4.36,5 = 14,6(g)
C%\(_{ddHCl}=\dfrac{ }{ }\)\(\dfrac{14,6.100}{280}=5,2\%\)
C2 :
2Al + 3H2SO4 -- > Al2(SO4)3 + 3H2
nH2 = 17,92/22,4 = 0,8 (mol)
mAl = (2/3.0,8 ) .27 = 14,4 (g)
mAl2(SO4)3 = (1/3 . 0,8 ) . 342 = 91,2 (g)
mH2SO4 = 0,8 . 98 = 78,4 (g)
\(C\%_{ddH_2SO_4}=\dfrac{78,4.100}{120}=65,33\%\)

n H2SO4=\(\dfrac{10\%.490}{2+32+16.4}=0,5mol\)
n Al2O3 =\(\dfrac{10,2}{27.2+16.3}=0,1mol\)
\(Al_2O_3+3H_2SO_4->Al_2\left(SO_4\right)_3+3H_2O\)
bđ 0,1............0,5
pư 0,1............0,3..................0,1
spu 0 ................0,2................0,1
=> sau pư gồm H2SO4 dư , Al2(S04)3 và H2O
m H2SO4 dư = \(0,2.\left(2+32+16.3\right)=19,6g\)
m Al2(SO4)3 = \(0,1\left(27.2+32.3+16.4.3\right)=34,2g\)
m dd = \(490+10,2=500,2g\)
% Al2(SO4)3 = \(\dfrac{34,2}{500,2}.100\sim6,84\%\)
% H2SO4 dư = \(\dfrac{19,6}{500,2}.100\sim3,92\%\)

a: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b: \(n_{H2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(\Leftrightarrow n_{Al}=0.1\left(mol\right)\)
\(m_{Al}=n_{Al}\cdot M_{Al}=0.1\cdot27=2.7\left(g\right)\)

2HCl +Ba(OH)2--->BaCl2 +2H2O
Ta có
n\(_{HCl}=0,4.0,1=0,04\left(mol\right)\)
Theo pthh
n\(_{Ba\left(OH\right)2}=\frac{1}{2}n_{HCl}=0,02\left(mol\right)\)
C\(_{M\left(Ba\left(OH\right)2\right)}=x=\frac{0,02}{0,2}=0,1\left(M\right)\)
Theo pthh
n\(_{BaCl2}=\frac{1}{2}n_{HCl}=0,02\left(mol\right)\)
C\(_{M\left(Ba\left(OH\right)2\right)}=\frac{0,02}{0,4+0,2}=0,033\left(M\right)\)
Chúc bạn học tốt
\(PTHH:Ba\left(OH\right)2+2HCl\rightarrow BaCl2+2H2O\)Đổi \(400ml=4l\)
Ta có : \(Cm=\frac{n}{v\text{dd}}\Rightarrow nHCl=0,1.4=0,4mol\)
\(\Rightarrow nBa\left(OH\right)2=0,2\left(mol\right)\)
CMBa(OH)2 = 0,4/0,2=2(M)
nBaCl = 0,2mol
=> CM= 0,2/0,4+0,2= 0,33 (M)

\(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1(mol)\\ Al_2O_3+3H_2SO_4\to Al_2(SO_4)_3+3H_2O\\ \Rightarrow n_{H_2SO_4}=0,3(mol)n_{Al_2(SO_4)_3}=0,1(mol)\\ \Rightarrow C\%_{H_2SO_4}=\dfrac{0,3.98}{300}.100\%=9,8\%\\ \Rightarrow C\%_{Al_2(SO_4)_3}=\dfrac{0,1.342}{10,2+300}.100\%=11,03\%\)

\(n_{Al}=\dfrac{1,35}{27}=0,05\left(mol\right)\\ 2Al+3H_2SO_4\rightarrow2Al_2\left(SO_4\right)_3+3H_2\\ n_{H_2SO_4}=n_{H_2}=\dfrac{3}{2}.0,05=0,075\left(mol\right)\\ n_{Al_2\left(SO_4\right)_3}=\dfrac{0,05}{2}=0,025\left(mol\right)\\ a,m_{Al_2\left(SO_4\right)_3}=342.0,025=8,55\left(g\right)\\ b,V_{H_2\left(đktc\right)}=0,075.22,4=1,68\left(l\right)\\ c,m_{H_2SO_4}=0,075.98=7,35\left(g\right)\)
\(n_{Al}=\dfrac{1,35}{27}=0,05mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,05 0,075 0,025 0,075
\(m_{Al_2\left(SO_4\right)_3}=0,025\cdot342=8,55g\)
\(V_{H_2}=0,075\cdot22,4=1,68l\)
\(m_{H_2SO_4}=0,075\cdot98=7,35g\)
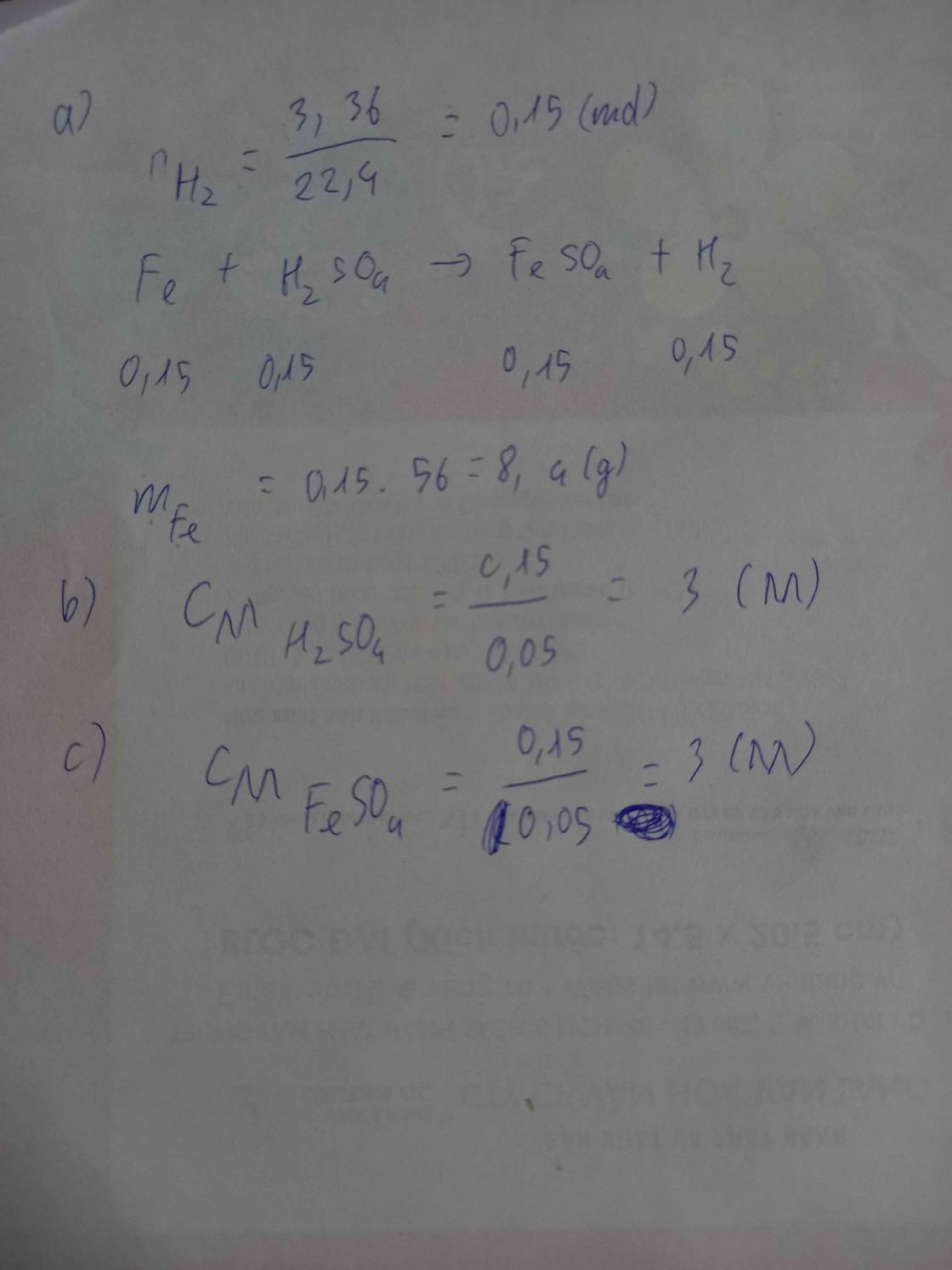
\(n_{Al}=\dfrac{1,35}{27}=0,05\left(mol\right)\)
\(n_{CuSO_4}=0,4.0,27=0,108\left(mol\right)\)
PTHH: 2Al + 3CuSO4 --> Al2(SO4)3 + 3Cu
_____0,05-->0,075------>0,025
=> \(\left\{{}\begin{matrix}C_{M\left(Al_2\left(SO_4\right)_3\right)}=\dfrac{0,025}{0,4}=0,0625M\\C_{M\left(CuSO_4\right)}=\dfrac{0,108-0,075}{0,4}=0,0825M\end{matrix}\right.\)