Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

$Mg + 2HCl \to MgCl_2 + H_2$
$Zn + 2HCl \to ZnCl_2 + H_2$
$n_{H_2} = \dfrac{2,912}{22,4} = 0,13(mol)$
$n_{HCl} = 2n_{H_2} = 0,13.2 = 0,26(mol)$
Bảo toàn khối lượng :
$m = m_{muối} + m_{H_2} - m_{HCl} = 14,4 + 0,13.2 - 0,26.36,5 = 5,17(gam)$

Câu 1:
Gọi \(\left\{{}\begin{matrix}n_{Cu}:x\left(mol\right)\\n_{Fe}:y\left(mol\right)\end{matrix}\right.\)
\(Cu+Cl_2\rightarrow CuCl_2\)
\(Fe+Cl_2\rightarrow FeCl_2\)
\(\left\{{}\begin{matrix}64x+56y=30,4\\2x+3y=1,2\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,3\\y=0,2\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Cu}=0,3.64=19,2\left(g\right)\\m_{Fe}=0,2.56=11,2\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Cu}=63,16\%\\\%m_{Fe}=36,84\%\end{matrix}\right.\)
BTNT Cl:
\(n_{AgCl}=2.n_{Cl2}=1,2\left(mol\right)\)
\(\Rightarrow m_{AgCl}=172,2\left(g\right)\)
Câu 2:
Gọi \(\left\{{}\begin{matrix}n_{Al}:x\left(mol\right)\\n_{Zn}:y\left(mol\right)\end{matrix}\right.\)
\(2Al+6HCl2\rightarrow AlCl_3+3H_2\)
x______________x________3x/2
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
y___________y_________y
\(m_{kl}=27x+65y=3,57\left(1\right)\)
\(m_{muoi}=133,5x+136y=12,09\left(2\right)\)
\(\left(1\right)+\left(2\right)\Rightarrow\left\{{}\begin{matrix}x=0,06\\y=0,03\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Al}=0,06.27=1,62\left(g\right)\\m_{Zn}=0,03.65=1,95\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%_{Al}=45,38\%\\\%_{Zn}=54,62\%\end{matrix}\right.\)
Bảo toàn e: \(n_{H2}=0,12\left(mol\right)\Rightarrow V=\frac{32}{12}=2,46\left(l\right)\)

1)
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\Rightarrow n_{HCl}=0,25.2=0,5\left(mol\right)\)
\(V=\dfrac{0,5}{0,5}=1\left(l\right)\)
2)
\(n_{NaCl}=\dfrac{5,85}{58,5}=0,1\left(mol\right)\); \(n_{AgNO_3}=\dfrac{34}{170}=0,2\left(mol\right)\)
PTHH: NaCl + AgNO3 --> NaNO3 + AgCl
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,2}{1}\) => NaCl hết, AgNO3 dư
PTHH: NaCl + AgNO3 --> NaNO3 + AgCl
0,1------------------------>0,1
=> mAgCl = 0,1.143,5 = 14,35 (g)





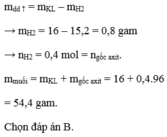
\(Zn+HCl\rightarrow ZnCl_2+H_2\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Gọi số mol H2 là a
Ta có: khối lượng dd tăng=m kim loại-mH2
\(\rightarrow1,79=1,94-m_{H2}\)
\(\rightarrow n_{H2}=0,075\left(mol\right)\)
\(\rightarrow n_{HCl}=2n_{H2}=0,075.2=0,15\left(mol\right)\)
Ta có nHCl=nCL=nAgCl=0,15
\(\rightarrow m_{AgCl}=0,15.143,5=21,525\left(g\right)\)