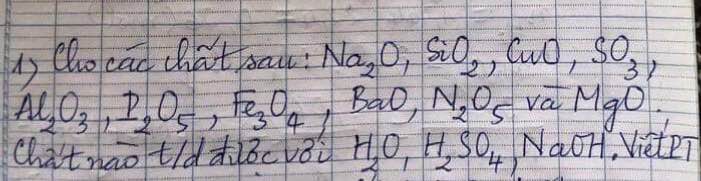Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


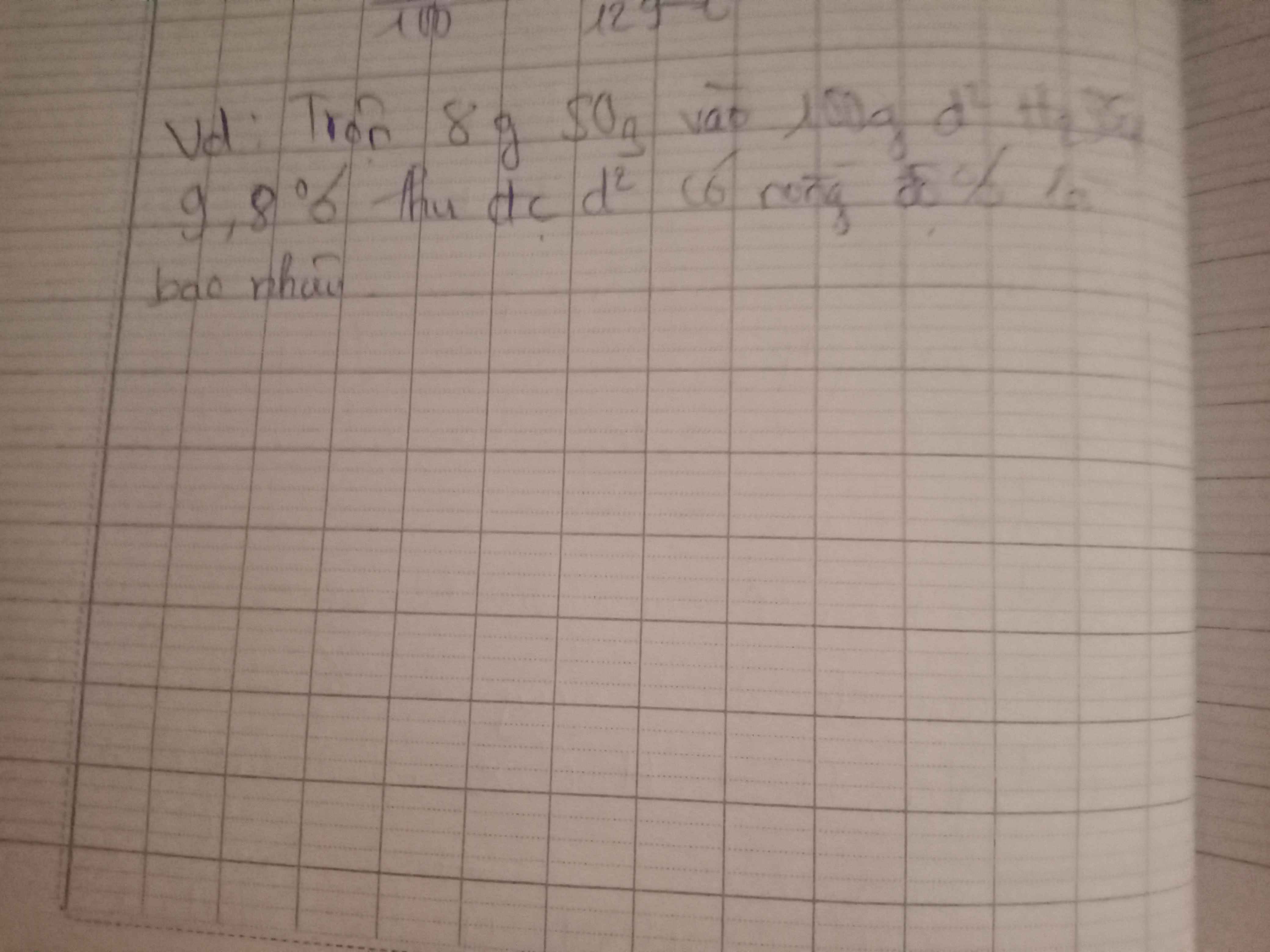
nSO3=8/80=0,1(mol)
pthh: SO3 + H2O -> H2SO4
nH2SO4=nSO3=0,1(mol) => mH2SO4(tạo sau)= 0,1.98=9,8(g)
mH2SO4(tổng)= 100.9,8% + 9,8=19,6(g)
mddH2SO4(sau)=8+100=108(g)
=>C%ddH2SO4(sau)= (19,6/108).100=18,148%

Câu 7:
a, \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
b, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,1.56}{10}.100\%=56\%\\\%m_{CuO}=44\%\end{matrix}\right.\)
c, \(n_{CuO}=\dfrac{10-0,1.56}{80}=0,055\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{Fe}+n_{CuO}=0,155\left(mol\right)\)
\(\Rightarrow C\%_{H_2SO_4}=\dfrac{0,155.98}{100}.100\%=15,19\%\)
d, Theo PT: \(\left\{{}\begin{matrix}n_{FeSO_4}=n_{Fe}=0,1\left(mol\right)\\n_{CuSO_4}=n_{CuO}=0,055\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{FeSO_4}=0,1.152=15,2\left(g\right)\\m_{CuSO_4}=0,055.160=8,8\left(g\right)\end{matrix}\right.\)
Câu 8:
a, \(CuCO_3+2HCl\rightarrow CuCl_2+CO_2+H_2O\)
b, \(n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Theo PT: \(n_{CuCO_3}=n_{CO_2}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CuCO_3}=\dfrac{0,15.124}{20}.100\%=93\%\\\%m_{CuCl_2}=7\%\end{matrix}\right.\)
c, \(n_{HCl}=2n_{CO_2}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\)

Bài 11:
\(PTHH:2A+Cl_2\rightarrow2ACl\\TheoĐLBTKL:\\ m_A+m_{Cl_2}=m_{ACl}\\ \Leftrightarrow 9,2+m_{Cl_2}=23,4\\ \Rightarrow m_{Cl_2}=23,4-9,2=14,2\left(g\right)\\ n_{Cl_2}=\dfrac{14,2}{71}=0,2\left(mol\right)\\ n_A=2.0,2=0,4\left(mol\right)\\ M_A=\dfrac{9,2}{0,4}=23\left(\dfrac{g}{mol}\right)\\ \Rightarrow A\left(I\right):Natri\left(Na=23\right)\)


d) Gọi x,y lần lượt là số mol Al, Fe
\(\left\{{}\begin{matrix}27x+56y=8,3\\1,5x+y=0,25\end{matrix}\right.\)
=> x=0,1 ; y=0,1
Kết tủa : Al(OH)3, Fe(OH)2
Bảo toàn nguyên tố Al: \(n_{Al\left(OH\right)_3}=n_{Al}=0,1\left(mol\right)\)
Bảo toàn nguyên tố Fe: \(n_{Fe\left(OH\right)_2}=n_{Fe}=0,1\left(mol\right)\)
=> \(m=0,1.78+0,1.90=16,8\left(g\right)\)
Nung kết tủa thu được chất rắn : Al2O3 và FeO
Bảo toàn nguyên tố Al: \(n_{Al_2O_3}.2=n_{Al}\Rightarrow n_{Al_2O_3}=0,05\left(mol\right)\)
Bảo toàn nguyên tố Fe: \(n_{FeO}=n_{Fe}=0,1\left(mol\right)\)
=> \(a=0,05.102+0,1.72=12,3\left(g\right)\)

Câu 6:
Gọi kim loại đó là \(R\)
\(\rightarrow Oxit:R_2O_3\)
Giả sử dd \(H_2SO_4\) phản ứng \(a\left(mol\right)\)
\(PTHH:R_2O_3+3H_2SO_4\rightarrow R_2\left(SO_4\right)_3+3H_2O\)
\(\left(mol\right)\) \(\dfrac{a}{3}\) \(a\) \(\dfrac{a}{3}\)
\(m_{ddH_2SO_4}=\dfrac{98a.100}{10}=980a\left(g\right)\)
\(C\%_{ddspu}=12,9\left(\%\right)\Leftrightarrow\dfrac{\left(2R+288\right).\dfrac{a}{3}}{\left(2R+48\right).\dfrac{a}{3}+980a}.100=12,9\\ \Leftrightarrow\dfrac{\dfrac{\left(2R+288\right)}{3}}{\dfrac{\left(2R+48\right)}{3}+980}.100=12,9\\ \Leftrightarrow R=56\left(Fe\right)\\ \rightarrow Oxit:Fe_2O_3\)
Câu 7:
\(a.n_{NaOH}=\dfrac{60.10\%}{40}=0,15\left(mol\right)\)
Đặt \(C\%_{HCl}=a\left(\%\right)\Rightarrow n_{HCl}=\dfrac{40a}{100.36,5}=\dfrac{4a}{365}\left(mol\right)\)
\(C\%_{NaCl}=5,85\%\Leftrightarrow\dfrac{m_{NaCl}}{60+40}.100=5,85\Leftrightarrow m_{NaCl}=5,85\left(g\right)\Leftrightarrow n_{NaCl}=0,1\left(mol\right)\)
\(PTHH:NaOH+HCl\rightarrow NaCl+H_2O\)
(mol) 0,1 0,1 0,1
Lúc này ta có: \(n_{HCl}=\dfrac{4a}{365}=0,1\Leftrightarrow a=9,125\left(\%\right)\)
Câu b làm tương tự!!!

Bài 1:
(1) \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
(2) \(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
(3) \(AlCl_3+3KOH\rightarrow3KCl+Al\left(OH\right)_3\downarrow\)
(4) \(Al\left(OH\right)_3+3HCl\rightarrow AlCl_3+3H_2O\)
(5) \(2Al\left(OH\right)_3\xrightarrow[]{t^o}Al_2O_3+3H_2O\)
(6) \(Al\left(OH\right)_3+NaOH\rightarrow NaAlO_2+2H_2O\)
(7) \(Al_2O_3+2NaOH\rightarrow2NaAlO_2+H_2O\)
(8) \(Al+NaOH+H_2O\rightarrow NaAlO_2+\dfrac{3}{2}H_2\uparrow\)
(9) \(2Al_2O_3\xrightarrow[criolit]{đpnc}4Al+3O_2\)
Bài 2:
PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
a_______a_______a_____a (mol)
\(Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\)
b_______b________b____b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}56a+24b=21,6\\a+b=\dfrac{11,2}{22,4}=0,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,3\\b=0,2\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,3\cdot56}{21,6}\cdot100\%\approx77,78\%\\\%m_{Mg}=22,22\%\end{matrix}\right.\)
Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{Mg\left(OH\right)_2}=n_{Mg}=0,2\left(mol\right)\\n_{Fe\left(OH\right)_2}=n_{Fe}=0,3\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{kết.tủa}=m_{Fe\left(OH\right)_3}+m_{Mg\left(OH\right)_2}=0,3\cdot107+0,2\cdot56=43,3\left(g\right)\)
Theo các PTHH: \(n_{H_2SO_4\left(p/ứ\right)}=0,5\left(mol\right)\) \(\Rightarrow n_{H_2SO_4\left(ban.đầu\right)}=0,5\cdot120\%=0,6\left(mol\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,6\cdot98}{10\%}=588\left(g\right)\)
Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{MgO}=n_{Mg}=0,2\left(mol\right)\\n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{chất.rắn}=m_{MgO}+m_{Fe_2O_3}=0,2\cdot40+0,15\cdot160=32\left(g\right)\)

Tác dụng với \(H_2O:\)\(Na_2O,SO_3,P_2O_5,BaO,N_2O_5,SiO_2\)
Tác dụng với \(H_2SO_4:Na_2O,CuO,Al_2O_3,Fe_3O_4,BaO,MgO\)
Tác dụng với NaOH: \(SiO_2,P_2O_5,N_2O_5,SO_3\)