
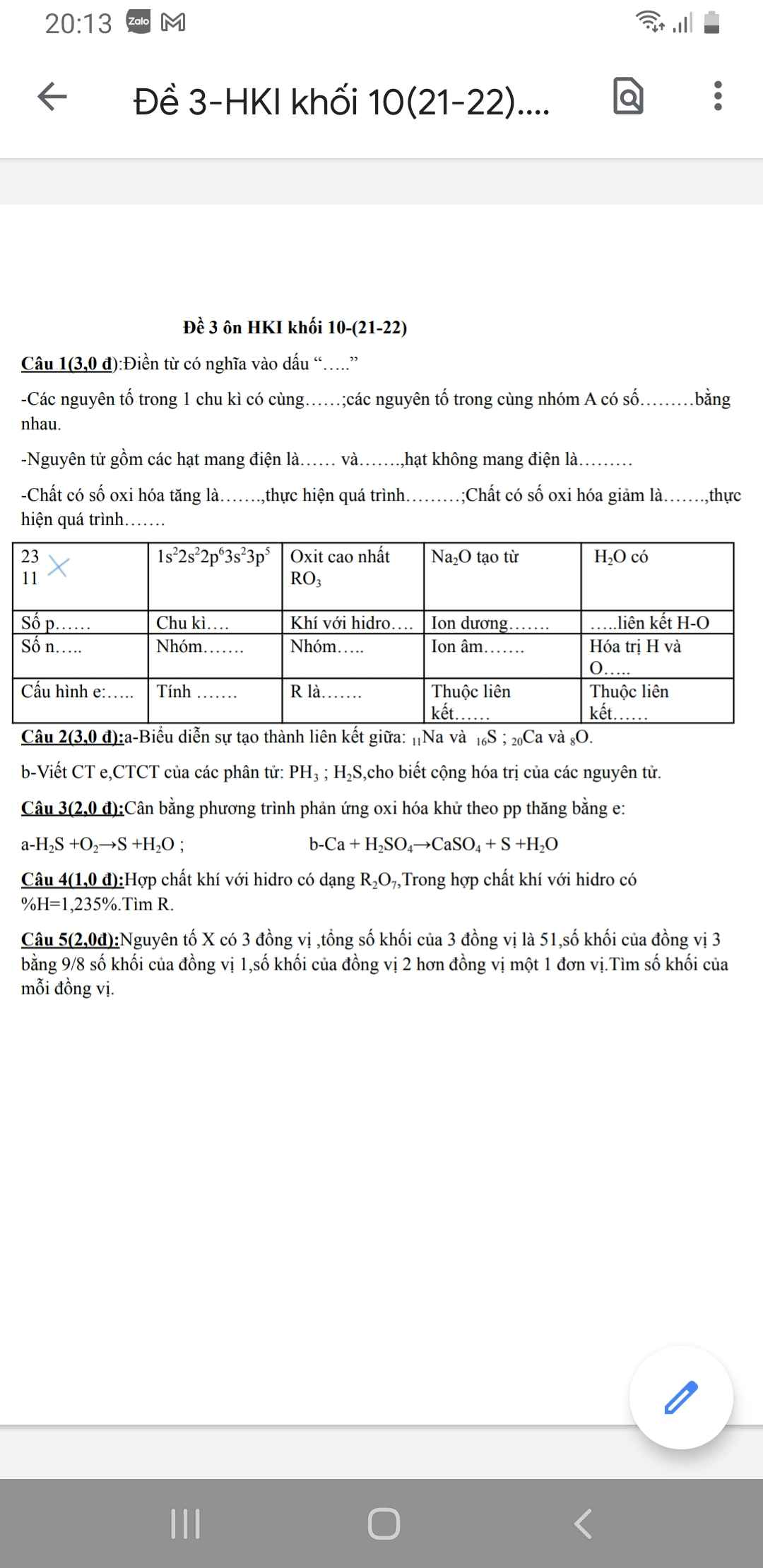
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(a,Ca+2HCl\rightarrow CaCl_2+H_2\left(1\right)\\ CaO+2HCl\rightarrow CaCl_2+H_2O\left(2\right)\\ Đặt:n_{Ca}=a\left(mol\right);n_{CaO}=b\left(mol\right)\\ \Rightarrow\left\{{}\begin{matrix}111a+111b=22,2\\22,4a=2,24\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\\ \Rightarrow\%m_{CaO}=\dfrac{0,1.56}{0,1.56+0,1.40}.100\approx52,83\%\\ \Rightarrow\%m_{Ca}=47,17\%\\ b,n_{HCl}=2.\left(a+b\right)=0,4\left(mol\right)\\ \Rightarrow m_{ddHCl}=\dfrac{0,4.36,5.100}{36,5}=40\left(g\right)\)

Bài 30:
\(Đặt:n_{KBr}=a\left(mol\right);n_{NaCl}=b\left(mol\right)\left(a,b>0\right)\\ KBr+AgNO_3\rightarrow AgBr\downarrow+KNO_3\\ NaCl+AgNO_3\rightarrow AgCl\downarrow+NaNO_3\\ \Rightarrow\left\{{}\begin{matrix}119a+58,5b=23,6\\188a+143,5b=47,5\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,2\end{matrix}\right.\\ a,m_{KBr}=0,1.119=11,9\left(g\right)\\ m_{NaCl}=0,2.58,5=11,7\left(g\right)\\ b,n_{AgNO_3}=a+b=0,3\left(mol\right)\\ \Rightarrow V_{ddAgNO_3}=\dfrac{0,3}{0,5}=0,6\left(l\right)=600\left(ml\right)\)



Bài 29 :
\(n_{CO2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Pt : \(CaO+2HCl\rightarrow CaCl_2+H_2O|\)
1 2 1 1
0,3 0,6 0,3
\(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O|\)
1 2 1 1 1
0,2 0,4 0,2 0,2
a) \(n_{CaCO3}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
\(m_{CaCO3}=0,2.100=20\left(g\right)\)
\(m_{CaO}=36,8-20=16,8\left(g\right)\)
0/0CaO = \(\dfrac{16,8.100}{36,8}=45,65\)0/0
0/0CaCO3 = \(\dfrac{20.100}{36,8}=54,35\)0/0
b) Có : \(m_{CaO}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
\(n_{HCl\left(tổng\right)}=0,6+0,4=1\left(mol\right)\)
\(C_{M_{ddHCl}}=\dfrac{1}{5}=0,2\left(M\right)\)
\(n_{CaCl2\left(tổng\right)}=0,3+0,2=0,5\left(mol\right)\)
\(C_{M_{CaCl2}}=\dfrac{0,5}{5}=0,1\left(M\right)\)
Chúc bạn học tốt
Bài 28:
\(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\\ K_2CO_3+2HCl\rightarrow2KCl+CO_2+H_2O\\ Đặt:n_{CaCO_3}=a\left(mol\right);n_{K_2CO_3}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}100a+138b=6,76\\a+b=\dfrac{2,64}{44}=0,06\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,04\\b=0,02\end{matrix}\right.\\ \%m_{CaCO_3}=\dfrac{0,02.100}{6,76}.100\approx29,586\%\\ \Rightarrow\%m_{K_2CO_3}\approx70,414\%\)


Dạng 4: Toán lượng dư:
Bài 1:nBr2=0,05x0,5=0,025 (mol)
PTHH: 2NaI+Br2→2NaBr+I2
0,025 0,05 (mol)
→ mNaBr=0,05.103= 5,15 (g)
Chúc em học giỏi

Bài 31:
Gọi số mol KHCO3, CaCO3 là x, y (mol)
\(n_{BaCO_3}=\dfrac{1,97}{197}=0,01\left(mol\right)\)
PTHH: KHCO3 + HCl --> KCl + CO2 + H2O
x----------------------->x
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
y----------------------------->y
Ba(OH)2 + CO2 --> BaCO3 + H2O
0,01<---0,01
=> x + y = 0,01
a = 100x + 100y = 1 (g)
Bài 32:
Gọi số mol K2CO3, CaCO3 là a, b (mol)
=> 138a + 100b = 11,9 (1)
PTHH: K2CO3 + 2HCl --> 2KCl + CO2 + H2O
a--------------->2a------>a
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
b-------------->b-------->b
=> \(a+b=\dfrac{2,24}{22,4}=0,1\) (2)
=> a = 0,05; b = 0,05
=> \(\left\{{}\begin{matrix}m_{KCl}=0,1.74,5=7,45\left(g\right)\\m_{CaCl_2}=0,05.111=5,55\left(g\right)\end{matrix}\right.\)
=> mmuối = 7,45 + 5,55 = 13 (g)
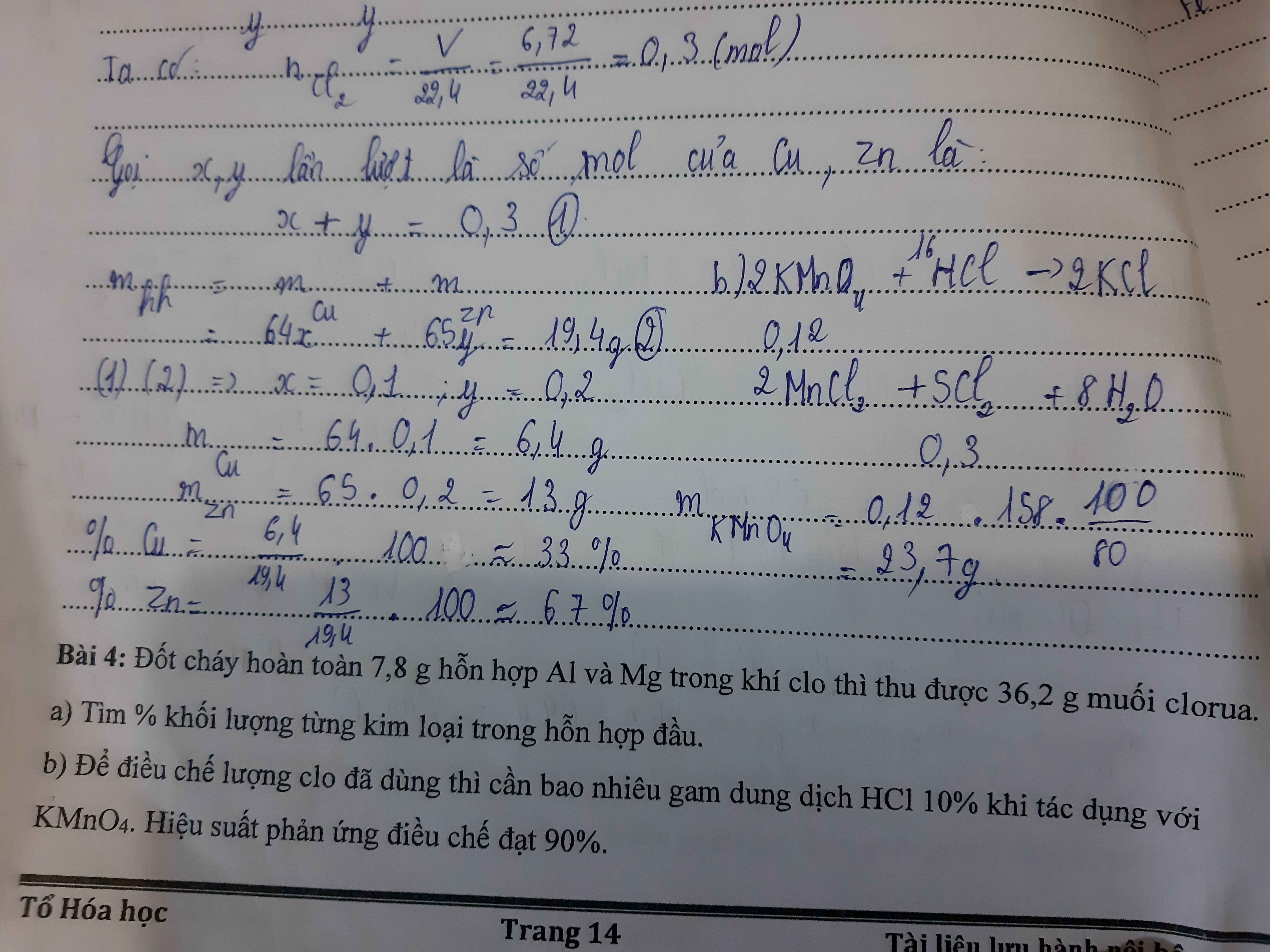
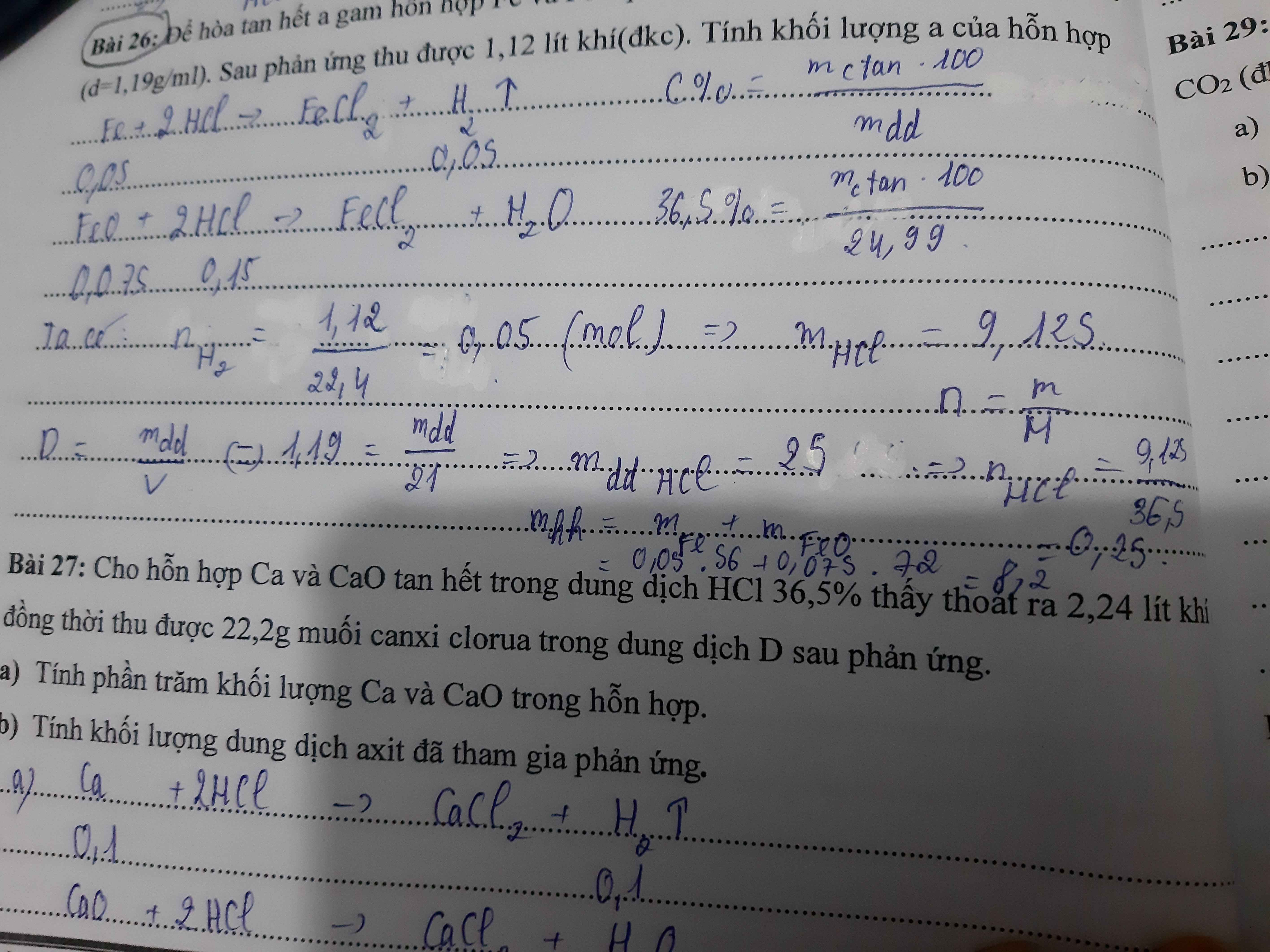









a.Theo định luật bảo toàn khối lượng, ta có:
\(m_{Cl_2}=36,2-7,8=28,4g\)
\(n_{Cl_2}=\dfrac{m}{M}=\dfrac{28,4}{71}=0,4mol\)
\(2Al+3Cl_2\rightarrow\left(t^o\right)2AlCl_3\)
2 3 2 ( mol )
\(2Mg+Cl_2\rightarrow\left(t^o\right)2MgCl_2\)
2 1 2 ( mol )
Gọi \(n_{Al}=x\)
\(n_{Mg}=y\)
\(\Rightarrow m_{Al}=27x\)
\(\Rightarrow m_{Mg}=24y\)
Ta có:
\(\left\{{}\begin{matrix}m_{hh}=27x+24y=7,8\\\dfrac{3}{2}x+\dfrac{1}{2}y=0,4\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}x=\dfrac{19}{75}\\y=\dfrac{1}{25}\end{matrix}\right.\)
\(\Rightarrow m_{Al}=27.\dfrac{19}{75}=6,84g\)
\(\Rightarrow m_{Mg}=24.\dfrac{1}{25}=0,96g\)
\(\%m_{Al}=\dfrac{6,84.100}{7.8}=87,7\%\)
\(\%m_{Mg}=100\%-87,7\%=12,3\%\)