Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{CaCl_2.6H_2O}=n_{CaCl_2}=\dfrac{5,475}{219}=0,025\left(mol\right)\)
Ta có :100ml H2O ~ 100g H2O
=> \(C\%_{CaCl_2}=\dfrac{0,025.111}{100+5,4750}.100=2,63\%\)

$n_{Na_2CO_3} = n_{Na_2CO_3.10H_2O} = \dfrac{28,6}{286} = 0,1(mol)$
$C_{M_{Na_2CO_3}} = \dfrac{0,1}{0,2} = 0,5M$
$m_{dd} = D.V = 200.1,05 = 210(gam)$
$C\%_{Na_2CO_3} = \dfrac{0,1.106}{210}.100\% = 5,05\%$

a) 300 ml nước ~ 300g nước
m dd = 25+300 = 325(g)
n CaCl2.6H2O = n CaCl2 = 25/219 = 0.11 (mol)
m CaCl2 = 0.11*111 = 12.21(g)
C%dd = 12.21/325*100% = 3.76%
V dd = 325/1.08 = 300.93(ml) = 0.3(l)
CM = 0.11/0.3 = 0.37M

a, \(Mg+2HCl\rightarrow MgCl_2+H_2\)
b, \(m_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\)
Theo PT: \(n_{MgCl_2}=n_{Mg}=0,3\left(mol\right)\Rightarrow m_{MgCl_2}=0,3.95=28,5\left(g\right)\)
c, \(n_{HCl}=2n_{Mg}=0,6\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,6}{0,2}=3\left(M\right)\)

\(n_{CuSO_4}=\dfrac{50}{250}=0.2\left(mol\right)\)
\(n_{FeSO_4}=\dfrac{27.8}{278}=0.1\left(mol\right)\)
\(C_{M_{CuSO_4}}=C_{M_{FeSO_4}}=\dfrac{0.1}{0.1964}=0.5\left(M\right)\)
\(m_{dd_A}=50+27.8+196.4=274.2\left(g\right)\)
\(C\%_{CuSO_4}=\dfrac{0.1\cdot160}{274.2}\cdot100\%=6.47\%\)
\(C\%_{FeSO_4}=\dfrac{0.1\cdot152}{274.2}\cdot100\%=5.54\%\)
\(n_{CuSO_4.5H_2O}=\dfrac{50}{250}=0,2\left(mol\right)\)
=> \(m_{CuSO_4}=0,2.160=32\left(g\right)\)
\(m_{H_2O}=0,2.5.18=18\left(g\right)\)
\(n_{FeSO_4.7H_2O}=\dfrac{27,8}{278}=0,1\left(mol\right)\)=> \(m_{FeSO_4}=0,1.152=15,2\left(g\right)\)
\(m_{H_2O}=0,1.7.18=12,6\left(g\right)\)
\(m_{dd}=196,4+50+27,8=274,2\left(g\right)\)
\(V_{dd}=\dfrac{196,4+18+12,6}{1000}=0,227\left(l\right)\)
=> \(CM_{CuSO_4}=\dfrac{0,2}{0,227}=0,72M\)
\(C\%_{CuSO_4}=\dfrac{32}{274,2}.100=11,67\%\)
\(CM_{FeSO_4}=\dfrac{0,1}{0,227}=0,44M\)
\(C\%_{CuSO_4}=\dfrac{15,2}{274,2}.100=5,54\%\)

a, PT: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
b, Ta có: \(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
Theo PT: \(n_{Zn}=n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow m_{Zn}=0,5.65=32,5\left(g\right)\)
\(\Rightarrow m_{CuO}=72,5-32,5=40\left(g\right)\)
c, Ta có: \(n_{CuO}=\dfrac{40}{80}=0,5\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{Zn}+n_{CuO}=1\left(mol\right)\)
\(\Rightarrow b=C_{M_{H_2SO_4}}=\dfrac{1}{2,5}=0,4M\)
c, Theo PT: \(\left\{{}\begin{matrix}n_{ZnSO_4}=n_{Zn}=0,5\left(mol\right)\\n_{CuSO_4}=n_{CuO}=0,5\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}C_{M_{ZnSO_4}}=\dfrac{0,5}{2,5}=0,2M\\C_{M_{CuSO_4}}=\dfrac{0,5}{2,5}=0,2M\end{matrix}\right.\)
Bạn tham khảo nhé!
a, \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\left(I\right)\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\left(II\right)\)
b, Theo PTHH(1) : \(n_{Zn}=n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow m_{Zn}=32,5\left(g\right)\)
\(\Rightarrow m_{CuO}=m_{hh}-m_{Zn}=40\left(g\right)\)
\(\Rightarrow n_{CuO}=\dfrac{m}{M}=0,5\left(mol\right)\)
c, Theo PTHH (1) và (2) : \(n_{H2SO4}=n_{CuO}+n_{Zn}=1\left(mol\right)\)
\(\Rightarrow C_{MH2SO4}=b=\dfrac{n}{V}=\dfrac{1}{2,5}=0,4M\)
d, ( Chắc là thể tích coi như không đổi )
Thấy sau phản ứng thu được A gồm \(0,5molZnSO_4,0,5molCuSO_4\)
\(\Rightarrow C_{MCuSO4}=C_{MZnSO4}=\dfrac{n}{V}=\dfrac{0,5}{2,5}=0,2M\)
Vậy ...
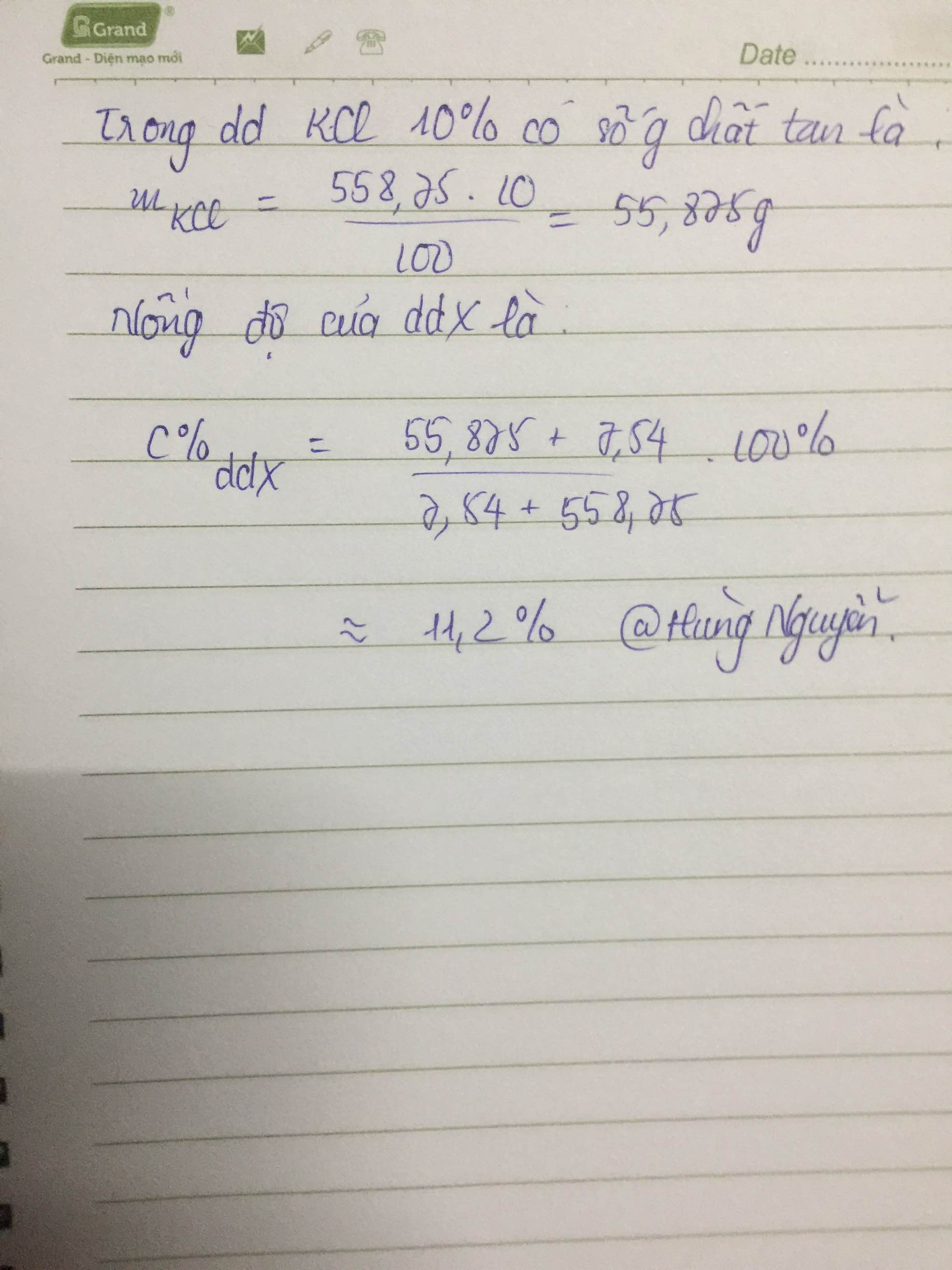
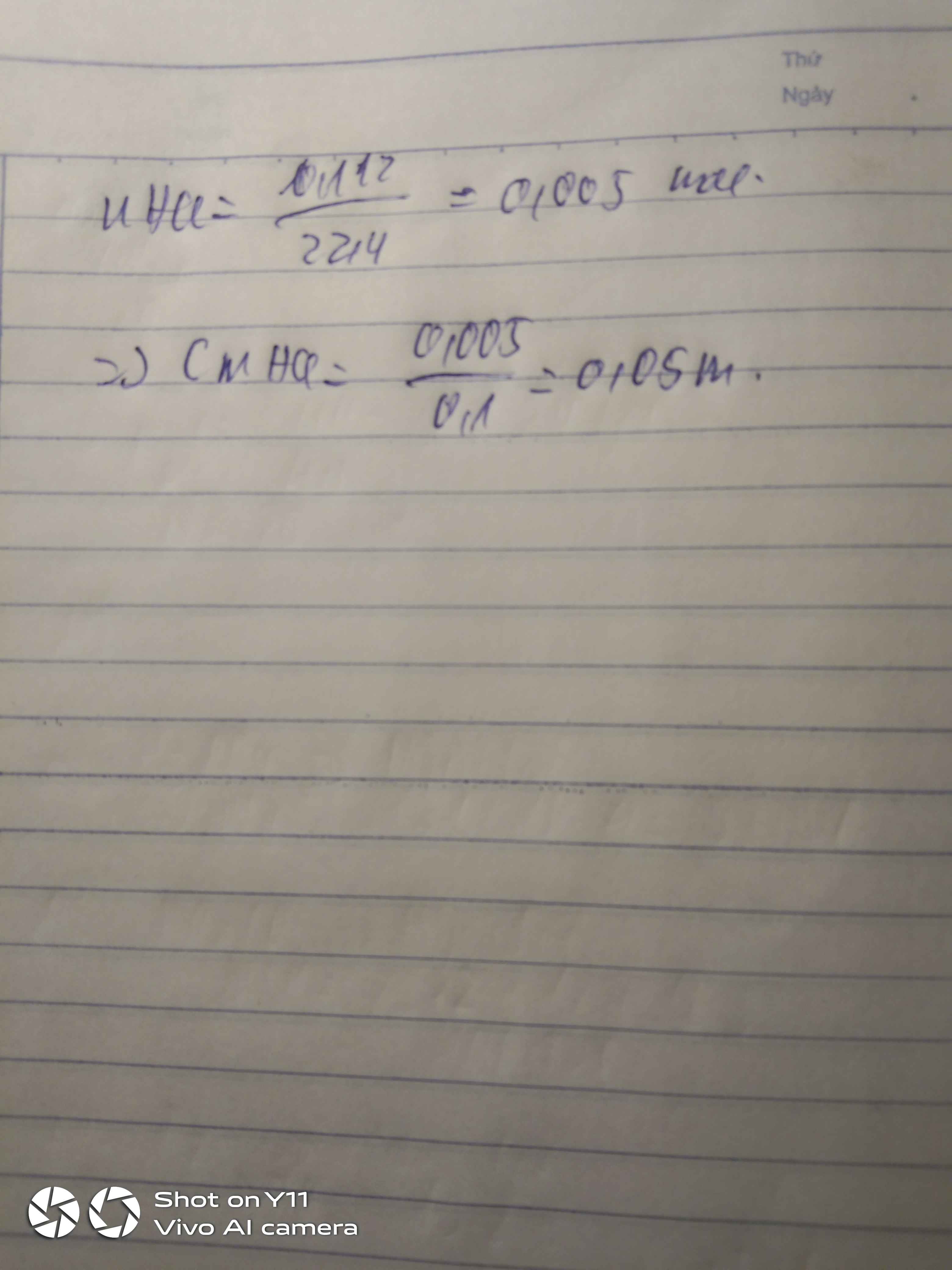
Ta có : \(n_{CaCl_2.6H_2O}=n_{CaCl_2}=\dfrac{5,475}{219}=0,025\left(mol\right)\)
=> CM CaCl2= \(\dfrac{0,025}{0,1}=0,25M\)