Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(C\%=\dfrac{30}{170}.100\%=17,647\%\)
\(V_{\text{dd}}=\left(30+170\right)1,1=220ml\)
\(n_{NaCl}=\dfrac{30}{58,5}=0,513mol\)
\(C_M=\dfrac{0,513}{0,22}=0,696M\)
\(C\%_{NaCl}=\dfrac{30}{170+30}.100\%=15\%\\ C_M=C\%.\dfrac{10D}{M}=10.\dfrac{10.1,1}{58,5}=1,88M\)

\(n_{P_2O_5}=\dfrac{14.2}{142}=0.1\left(mol\right)\)
\(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
\(0.1............................0.2\)
\(m_{H_3PO_4}=0.2\cdot98=19.6\left(g\right)\)
\(m_{dd_{H_3PO_4}}=14.2+185.8=200\left(g\right)\)
\(C\%H_3PO_4=\dfrac{19.6}{200}\cdot100\%=9.8\%\)
\(V_{dd_{H_3PO_4}}=\dfrac{200}{1.1}=181.8\left(ml\right)=0.1818\left(l\right)\)
\(C_{M_{H_3PO_4}}=\dfrac{0.2}{0.1818}=1.1\left(M\right)\)
Ta có: \(n_{P_2O_5}=\dfrac{14,2}{142}=0,1\left(mol\right)\)
PT: \(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
____0,1_____________0,2 (mol)
\(\Rightarrow m_{H_3PO_4}=0,2.98=19,6\left(g\right)\)
Có: m dd sau pư = mP2O5 + mH2O = 200 (g)
\(\Rightarrow C\%_{H_3PO_4}=\dfrac{19,6}{200}.100\%=9,8\%\)
Có: V dd sau pư = \(\dfrac{200}{1,1}=\dfrac{2000}{11}\left(ml\right)=\dfrac{2}{11}\left(l\right)\)
\(\Rightarrow C_{M_{H_3PO_4}}=\dfrac{0,2}{\dfrac{2}{11}}=1,1M\)
Bạn tham khảo nhé!

Sửa đề: 9,2 gam Na
\(a,n_{Na_2O}=\dfrac{9,2}{23}=0,4\left(mol\right)\)
PTHH: \(Na_2O+H_2O\rightarrow2NaOH\)
0,4------------------>0,8
\(\rightarrow C_{M\left(NaOH\right)}=\dfrac{0,8}{0,5}=1,6M\)
\(b,n_{K_2O}=\dfrac{37,6}{94}=0,4\left(mol\right)\)
PTHH: \(K_2O+H_2O\rightarrow2KOH\)
0,4----------------->0,8
\(\rightarrow C\%_{KOH}=\dfrac{0,8.56}{362,4+37,6}.100\%=11,2\%\)

a) \(C\%=\dfrac{m_{KCl}}{m_{ddKCl}}.100\%=\dfrac{10}{300}.100\%\approx3,3\%\)
b) Đổi: \(1500ml=1,5l\)
\(C_{MCuSO_4}=\dfrac{n}{V}=\dfrac{3}{1,5}=2M\)

\(m_{H_2O}=\dfrac{171,3}{1}=171,3\left(g\right)\\ m_{dd.thu.được}=m_{tinh.thể}+m_{H_2O}=28,7+171,3=200\left(g\right)\\ n_{ZnSO_4}=n_{tinh.thể}=\dfrac{28,7}{161+7.18}=0,1\left(mol\right)\\ V_{H_2O\left(dd.thu.được\right)}=\dfrac{200-0,1.161}{1000}=0,1839\left(l\right)\\ C_{MddZnSO_4}=\dfrac{0,1}{0,1839}\approx0,5438\left(M\right)\)

Ta có: \(n_{Na_2CO_3}=n_{Na_2CO_3.10H_2O}=\dfrac{38,61}{286}=0,135\left(mol\right)\)
m dd sau pư = 38,61 + 256 = 294,61 (g)
\(\Rightarrow C\%_{Na_2CO_3}=\dfrac{0,135.106}{294,61}.100\%\approx4,86\%\)
Có: \(V_{ddsaupư}=\dfrac{294,61}{1,156}\approx254,85\left(ml\right)\approx0,255\left(l\right)\)
\(\Rightarrow C_{M_{Na_2CO_3}}=\dfrac{0,135}{0,255}\approx0,53M\)
Bạn tham khảo nhé!
Gọi số mol của Na2CO3 là a (mol) \(\Rightarrow n_{H_2O\left(phân.tử\right)}=10a\left(mol\right)\)
\(\Rightarrow106a+18\cdot10a=38,61\) \(\Leftrightarrow a=0,135\left(mol\right)\)
\(\Rightarrow C\%_{Na_2CO_3}=\dfrac{0,135\cdot106}{38,61+256}\cdot100\%\approx4,86\%\)
Mặt khác: \(V_{ddNa_2CO_3}=\dfrac{38,61+256}{1,156}\approx254,41\left(ml\right)\) \(\Rightarrow C_{M_{Na_2CO_3}}=\dfrac{0,135}{0,25441}\approx0,53\left(M\right)\)
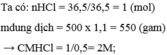
\(n_{HCl}=\dfrac{36,5}{36,5}=1\left(mol\right)\)
\(\Rightarrow C_{MddHCl}=\dfrac{1}{0,5}=2M\)
\(m_{ddHCl}=500.1,1=550\left(g\right)\)
\(\Rightarrow C\%ddHCl=\dfrac{36,5}{550}.100\%\approx6,6\%.\)