Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(NaOH+HCl->NaCl+H_2O\\ 2NaOH+H_2SO_4->Na_2SO_4+2H_2O\\ a.V=\dfrac{0,1.1}{2}=0,05\left(L\right)\\ b.m_{ddH_2SO_4}=\dfrac{0,1.1.98}{2.0,1}=49\left(g\right)\)

\(n_{H_2SO_4}=1.0,2=0,2(mol)\\ PTHH:2NaOH+H_2SO_4\to Na_2SO_4+2H_2O\\ a,n_{NaOH}=0,4(mol);n_{Na_2SO_4}=0,2(mol)\\ \Rightarrow \begin{cases} m_{Na_2SO_4}=0,2.142=28,4(g)\\ m_{dd_{NaOH}}=\dfrac{0,4.40}{20\%}=80(g) \end{cases}\\ b,2KOH+H_2SO_4\to K_2SO_4+2H_2O\\ \Rightarrow n_{KOH}=0,4(mol)\\ \Rightarrow m_{dd_{KOH}}=\dfrac{0,4.56}{5,6\%}=400(g)\\ \Rightarrow V_{dd_{KOH}}=\dfrac{400}{1,045}=382,78(ml)\)
Bước 1: nH2SO4 = VH2SO4 . CM H2SO4= 0,2 . 1 = 0,2mol
Bước 2:
PTHH: 2NaOH + H2SO4 → Na2SO4 + H2O
2 mol 1 mol
? mol 0,2mol
nNaOH=0,2.21=0,4mol.nNaOH=0,2.21=0,4mol.
m NaOH= n NaOH.MNaOH = 0,4 . (23 + 16 + 1) = 16g
Bước 3: C% = mNaOH : m dd NaOH => mdd NaOH = mNaOH : C% = 16 : 20% = 80g

nH2SO4=0,02.1=0,02(ol)
a) PTHH: 2 NaOH + H2SO4 -> Na2SO4 + 2 H2O
0,04____________0,02____0,02(mol)
mNaOH=0,04.40= 1,6(g)
=>mddNaOH= (1,6.100)/20= 8(g)
b) PTHH: H2SO4 + 2 KOH -> K2SO4 + 2 H2O
0,2____________0,04(mol)
=>mKOH=0,04.56=2,24(g)
=>mddKOH= (2,24.100)/5,6=40(g)
=>VddKOH= mddKOH/DddKOH= 40/1,045=38,278(ml)

\(m_{NaOH}=\frac{50.10}{100}=5\left(g\right)\Rightarrow n_{NaOH}=\frac{5}{40}=0,125\left(mol\right)\)
\(PTHH:\text{ }H_2SO_4+2NaOH\rightarrow Na_2SO_4+2H_2O\)
\(Theo\text{ PT: }n_{H_2SO_4}=\frac{1}{2}n_{NaOH}=\frac{1}{2}.0,125=0,0625\left(mol\right)\)
\(Vdd_{H_2SO_4}=\frac{n_{H_2SO_4}}{C_M}=\frac{0,0625}{0,5}=0,125\left(l\right)=125\text{ }ml\)
\(m_{NaOH}=\frac{50.10}{100}\)=5 g
\(n_{NaOH}=\frac{5}{40}=0,125\left(mol\right)\)
PTHH: H2SO4+2NaOH\(\rightarrow\)Na2SO4+2H2O
0,0625 \(\leftarrow\)0,125( mol)
VẬY \(V_{H2SO4}=\frac{0,0625}{0,5}=0,125\left(l\right)\)
ta có: 1l=1000ml\(\Rightarrow\) 0,125(l)=125ml
chúc bạn học tốt like nha

nHCl= 1,5.0,2=0,3(mol); nH2SO4= 1.0,2=0,2(mol)
PTHH: NaOH + HCl -> NaCl + H2O
0,3_________0,3(mol)
2 NaOH + H2SO4 -> Na2SO4 + 2 H2O
0,4_______0,2(mol)
=>> nNaOH(tổng)=0,3+0,4=0,7(mol)
=> VddNaOH= 0,7/0,2=0,35(l)=350(ml)
=> CHỌN C

Câu 3 :
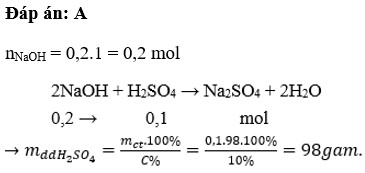
\(n_{NaOH}=0,2.1=0,2\left(mol\right)\)
Pt : \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
\(n_{H2SO4}=\dfrac{1}{2}n_{NaOH}=0,1\left(mol\right)\Rightarrow m_{ddH2SO4}=\dfrac{0,1.98}{10\%}.100\%=98\left(g\right)\)
\(n_{H_2SO_4}=0,15.1=0,15\left(mol\right)\)
PTHH: \(H_2SO_4+2NaOH\rightarrow Na_2SO_4+2H_2O\)
________0,15------->0,3_________________________(mol)
=> \(m_{NaOH}=0,3.40=12\left(g\right)\)
=> \(m_{ddNaOH}=\dfrac{12.100}{10}=120\left(g\right)\)
nH2SO4=1.0,15=0,15(mol)
2NaOH + H2SO4 \(\rightarrow\) Na2SO4 + 2H2O
0,3 \(\leftarrow\) 0.15 (mol)
=> mNaOH= 0,3.40= 12(g)
=> mdung dịch NaOH 10%= \(\dfrac{12.100\%}{10\%}\)= 120(g)