Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{NaCl\left(lt\right)}=\dfrac{8.775}{58.5}=0.15\left(mol\right)\)
\(2Na+Cl_2\underrightarrow{^{t^0}}2NaCl\)
\(0.15..................0.15\)
\(m_{Na\left(tt\right)}=\dfrac{0.15\cdot23}{75\%}=4.6\left(g\right)\)
nNaCl=\(\dfrac{8,775}{58,5}=0,15\left(mol\right)\)
PTHH 2Na+Cl2---->2NaCl
---------0,15------------0,15
=>nNa(tt)=\(\dfrac{0,15}{75}.100=0,2\left(mol\right)\)
=>mNa(tt)=0,2.23=4,6(g)

a. \(2KMnO_4\rightarrow K_2MnO_4+MnO_2+O_2\)
\(n_{KMnO_4}=0,6mol\)
\(\rightarrow n_{O_2}=\frac{1}{2}n_{KMnO_4}=0,3mol\)
\(\rightarrow V_{O_2}=6,72l\)
\(V_{O_2\text{thực}}=\frac{6,72.75}{100}=5,04l\)
b. \(2KMnO_4\rightarrow K_2MnO_4+MnO_2+O_2\)
\(n_{O_2}=1,5mol\)
\(\rightarrow n_{KMnO_4}=2n_{O_2}=3mol\)
\(\rightarrow m_{KMnO_4\text{cần}}=\frac{474.100}{80}=592,5g\)

Phương trình hóa học CaCO3 → CaO + CO2.
a) nCaO = 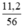 = 0,2 mol.
= 0,2 mol.
Theo PTHH thì nCaCO3 = nCaO = 0,2 (mol)
b) nCaO = 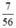 = 0,125 (mol)
= 0,125 (mol)
Theo PTHH thì nCaCO3 = nCaO = 0,125 (mol)
mCaCO3 = M.n = 100.0,125 = 12,5 (g)
c) Theo PTHH thì nCO2 = nCaCO3 = 3,5 (mol)
VCO2 = 22,4.n = 22,4.3,5 = 78,4 (lít)
d) nCO2 = 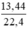 = 0,6 (mol)
= 0,6 (mol)
Theo PTHH nCaO = nCaCO3 = nCO2 = 0,6 (mol)
mCaCO3 = n.M = 0,6.100 = 60 (g)
mCaO = n.M = 0,6.56 = 33,6 (g)

\(n_{O_2} = \dfrac{6,72}{22,4} = 0,3(mol)\)
\(2KClO_3 \xrightarrow{t^o} 2KCl + 3O_2\)
Theo PTHH :
\(n_{KClO_3\ phản\ ứng} = \dfrac{2}{3}n_{O_2} = 0,2(mol)\\ \Rightarrow n_{KClO_3\ cần\ dùng} = \dfrac{0,2}{70\%} = \dfrac{2}{7}(mol)\\ \Rightarrow m_{KClO_3\ cần\ dùng} = \dfrac{2}{7}.122,5 = 35(gam)\)

a) \(n_{Cu\left(NO_3\right)_2}=\dfrac{282}{188}=1,5\left(mol\right)\)
=> \(n_{Cu\left(NO_3\right)_2\left(pư\right)}=\dfrac{1,5.90}{100}=1,35\left(mol\right)\)
PTHH: 2Cu(NO3)2 --to--> 2CuO + 4NO2 + O2
______1,35------------>1,35------------->0,675
=> mCuO = 1,35.80 = 108(g)
=> VO2 = 0,675.22,4 = 15,12 (l)
b) Gọi số mol Cu(NO3)2 cần nung là a (mol)
=> \(n_{Cu\left(NO_3\right)_2\left(pư\right)}=\dfrac{90a}{100}=0,9a\left(mol\right)\)
PTHH: 2Cu(NO3)2 --to--> 2CuO + 4NO2 + O2
______0,9a---------------------->1,8a--->0,45a
=> (1,8a+0,45a).22,4 = 5
=> a = 0,0992 (mol)
=> \(m_{Cu\left(NO_3\right)_2}=0,0992.188=18,6496\left(g\right)\)

Ta có: \(n_{ZnCl_2}=\dfrac{27,2}{136}=0,2\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Theo PT: \(n_{Zn\left(LT\right)}=n_{ZnCl_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Zn\left(LT\right)}=0,2.65=13\left(g\right)\)
Mà: H = 75% \(\Rightarrow m_{Zn\left(TT\right)}=\dfrac{13}{75\%}=\dfrac{52}{3}\left(g\right)\)

câu 5
nKMnO4=\(\dfrac{31,6.98\%}{158}\)=0,196(mol)
2KMnO4−to→K2MnO4+MnO2+O2
nO2(lt)=\(\dfrac{1}{2}\)nKMnO4=0,098(mol)
Vìhaohụt5%
⇒VO2(tt)=0,098.95%.22,4=2,08544(l)

\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH :
\(2Na+2H_2O\rightarrow2NaOH+H_2\uparrow\)
1 0,5
\(b,m_{NaOH}=1.40=40\left(g\right)\)
\(c,H_2+O_2\underrightarrow{t^o}2H_2O\)
0,5 0,5
\(V_{O_2}=0,5.22,4=11,2\left(l\right)\)
\(V_{kk}=11,2.5=56\left(l\right)\)

\(m_{KMnO_4}=31,6.98\%=30,968g\)
\(m_{KMnO_4}=30,968.95\%=29,4196g\)
\(n_{KMnO_4}=\dfrac{m_{KMnO_4}}{M_{KMnO_4}}=\dfrac{29,4196}{158}=0,1862mol\)
\(2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\)
0,1862 0,0931 ( mol )
\(V_{O_2}=n_{O_2}.22,4=0,0931.22,4=2,08544l\)
\(n_{KMnO_4}=\dfrac{31,6.98\%}{158}=0,196\left(mol\right)\\ 2KMnO_4-^{t^o}\rightarrow K_2MnO_4+MnO_2+O_2\\ n_{O_2\left(lt\right)}=\dfrac{1}{2}n_{KMnO_4}=0,098\left(mol\right)\\ Vìhaohụt5\%\\ \Rightarrow V_{O_2\left(tt\right)}=0,098.95\%.22,4=2,08544\left(l\right)\)
PTHH: 2Na+Cl2\(\rightarrow\)2NaCl
nNaCl=\(\dfrac{8,775}{58.5}=0,15\left(mol\right)\)
nếu hiệu suất là 100% thì nNaCl=0,15/\(\dfrac{75}{100}\)=0,2(mol)
theo PTHH: nNa=nNaCl=0,2
\(\rightarrow\)mNa=0,2.23=4,6(g)
theo PTHH: nCl2=\(\dfrac{1}{2}\)nNaCl=0,1
\(\rightarrow\)VCl2=0,1.22,4=2,24(l)
2Na +Cl2 --> 2NaCl
nNaCl=8,775/58,5=0,15(mol)
theo PTHH : nCl2=1/2nNaCl=0,075(mol)
mà H=75% =>nCl2(thực tế)=0,075/75.100=0,1(mol)
=>mCl2=0,1.71=7,1(g)
nNa=nNaCl=0,15(mol)
mà H=75%=>nNa(thực tế )=0,15/75.100=0,2(mol)
=>mNa=0,2.23=4,6(g)