Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a
\(Cu+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2Ag\downarrow\)
0,05 --> 0,1-----------> 0,05------>0,1
b
\(n_{Cu}=\dfrac{3,2}{64}=0,05\left(mol\right)\)
\(m_{Cu\left(NO_3\right)_2}=0,05.188=9,4\left(g\right)\)
c
\(a=m_{Ag}=108.0,1=10,8\left(g\right)\)
d
\(V_{AgNO_3}=\dfrac{0,1}{0,5}=0,2\left(l\right)\)

\(a/2Al+3H_2SO_4\xrightarrow[]{}Al_2\left(SO_4\right)_3+3H_2\)
\(b/30ml=0,03l\\ n_{H_2SO_4}=0,5.0,03=0,0015\left(mol\right)\\ n_{Al}=\dfrac{0,0015.2}{3}=0,001\left(mol\right)\\ m_{Al}=0,001.27=0,027\left(g\right)\\ n_{Al_2\left(SO_4\right)_3}=\dfrac{0,0015}{2}=0,00075\left(mol\right)\\ m_{Al_2\left(SO_4\right)_3}=0,00075.342=0,2565\left(g\right)\)
\(c/n_{H_2}=\dfrac{0,0015.3}{3}=0,0015\left(mol\right)\\ V_{H_2}=0,0015.24,79=0,037185\left(l\right)\)
\(a.2Al+3H_2SO_4->Al_2\left(SO_4\right)_3+3H_2\\ b.n_{Al}=1,5.0,5.0,03=0,0375mol\\ m_{Al}=0,0375.27=1,0125g\\ m_{Al_2\left(SO_4\right)_3}=342\cdot\dfrac{1}{3}\cdot0,03\cdot0,5=1,71g\\V_{H_2}=24,79.0,5.0,03=0,37185L\)

\(n_{Zn}=\dfrac{9,75}{65}=0,15\left(mol\right)\\
pthh:Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
0,15 0,15 0,15 0,15
\(V_{H_2}=0,15.22,4=3,36L\\
m_{H_2SO_4}=0,15.98=14,7\left(g\right)\\
m_{ZnSO_4}=161.0,15=24,15g\\
\)
\(n_{CuO}=\dfrac{6}{80}=0,075\left(mol\right)\\
pthh:CuO+H_2\underrightarrow{t^o}Cu+H_2O\\
LTL:0,075< 0,15\)
=> H2 dư
\(n_{Cu}=n_{CuO}=0,075\left(mol\right)\\
m_{Cu}=0,075.64=4,8g\)

Theo gt ta có: \(n_{Cu}=\dfrac{12,8}{64}=0,2\left(mol\right)\)
\(1Bar=0,9869atm\)
PTHH: \(2Cu+O_2\rightarrow2CuO\)
Ta có: \(n_{CuO}=n_{Cu}=0,2\left(mol\right)\Rightarrow a=m_{CuO}=16\left(g\right)\)
\(n_{O_2}=\dfrac{1}{2}.n_{Cu}=0,1\left(mol\right)\Rightarrow V=\dfrac{n.R.T}{p}=\dfrac{0,1.\dfrac{22,4}{273}.\left(273+20\right)}{0,9869}=2,436\left(l\right)\)

\(Mg+2HCl\rightarrow MgCl_2+H_2\)
0,05 -->0,1----->0,05----->0,05
b
\(n_{Mg}=\dfrac{1,2}{24}=0,05\left(mol\right)\)
\(m_{MgCl_2}=0,05.95=4,75\left(g\right)\)
c
\(V_{H_2}=0,05.24,79=1,2395\left(l\right)\)
d
\(V_{HCl}=\dfrac{0,1}{2}=0,05\left(l\right)\)

\(a/2Al+6HCl\xrightarrow[]{}2AlCl_3+3H_2\\ b/n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ n_{AlCl_3}=n_{Al}=0,2mol\\ m_{AlCl_3}=0,2.133,5=26,7\left(g\right)\\ c/higro\Rightarrow hydrogen\\ n_{H_2}=\dfrac{0,2.3}{2}=0,3\left(mol\right)\\ V_{H_2}=0,3.24,79=7,437\left(l\right)\\ d/n_{HCl}=\dfrac{0,2.6}{2}=0,6\left(mol\right)\\ V_{HCl}=\dfrac{0,6}{2}=0,3\left(l\right)\)

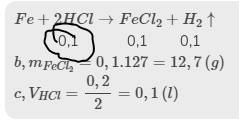
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
0,2 0,1 0,1
\(b,m_{FeCl_2}=0,1.127=12,7\left(g\right)\)
\(c,V_{HCl}=\dfrac{0,2}{2}=0,1\left(l\right)\)
\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
a \(Zn+CuSO_4\rightarrow ZnSO_4+Cu\)
0,1 ---> 0,1------------------> 0,1
b
\(a=m_{Cu}=0,1.64=6,4\left(g\right)\)
c
\(V_{CuSO_4}=\dfrac{0,1}{0,5}=0,2\left(l\right)\)