Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
a) Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,15 0,3 0,15
b) \(n_{Fe}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_{Fe}=0,15.56=8,4\left(g\right)\)
c) \(n_{HCl}=\dfrac{0,15.2}{1}=0,3\left(mol\right)\)
50ml = 0,05l
\(C_{M_{ddHCl}}=\dfrac{0,3}{0,05}=6\left(M\right)\)
Chúc bạn học tốt

a)
$Fe + 2HCl \to FeCl_2 + H_2$
b) Theo PTHH : $n_{Fe} = n_{H_2} = \dfrac{3,36}{22,4} = 0,15(mol)$
$m_{Fe} = 0,15.56 = 8,4(gam)$
c) $n_{HCl} = 2n_{H_2} = 0,3(mol)$
$\Rightarrow C_{M_{HCl}} = \dfrac{0,3}{0,05} = 6M$
d) $2NaOH + H_2SO_4 \to Na_2SO_4 + 2H_2O$
$n_{H_2SO_4} = \dfrac{1}{2}n_{NaOH} = 0,25(mol)$
$m_{dd\ H_2SO_4} = \dfrac{0,25.98}{20\%} = 122,5(gam)$
$V_{dd\ H_2SO_4} = \dfrac{122,5}{1,14} = 107,5(ml)$

a) \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
____0,15<--0,3<-------------0,15
=> mFe = 0,15.56 = 8,4 (g)
b) \(C_{M\left(ddHCl\right)}=\dfrac{0,3}{0,05}=6M\)

nH2=3.36/22.4=0.15 mol
a) PT: Fe + 2HCl ----> FeCl2 + H2
0.15 0.3 0.15
b)mFe=0.15*56=8.4g
c)CMHCl= 0.3*0.05=6 M
Chúc em học tốt!!!
Fe+2HCl->FeCl2+H2
nH2=0.15(mol)
Theo pthh nFe=nH2->nFe=0.15(mol)
mFe phản ứng:0.15*56=8.4(g)
nHCl=2nH2->nHCl=0.3(mol)
CM=0.3:0.05=6 M

\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
\(n_{Fe}=n_{H_2}=0,15\left(mol\right)\)
\(m_{Fe}=0,15.56=8,4\left(g\right)\)
\(n_{HCl}=2n_{H_2}=0,3\left(mol\right)\)
\(50ml=0,05l\)
\(C_{M_{ddHCl}}=\dfrac{0,3}{0,05}=6\left(M\right)\)

\(Fe+H_2SO_4 \to FeSO_4+H_2\\ n_{H_2}=0,15(mol)\\ a/\\ n_{Fe}=n_{H_2}=0,15(mol)\\ m_{Fe}=0,15.56=8,4(g)\\ b/\\ n_{H_2SO_4}=n_{H_2}=0,15(mol)\\ CM_{H_2SO_4}=\dfrac{0,15}{2}=0,75M c/\\ n_{FeSO_4}=n_{H_2}=0,15(mol)\\ CM_{FeSO_4}=\dfrac{0,15}{0,2}=0,75M\\\)
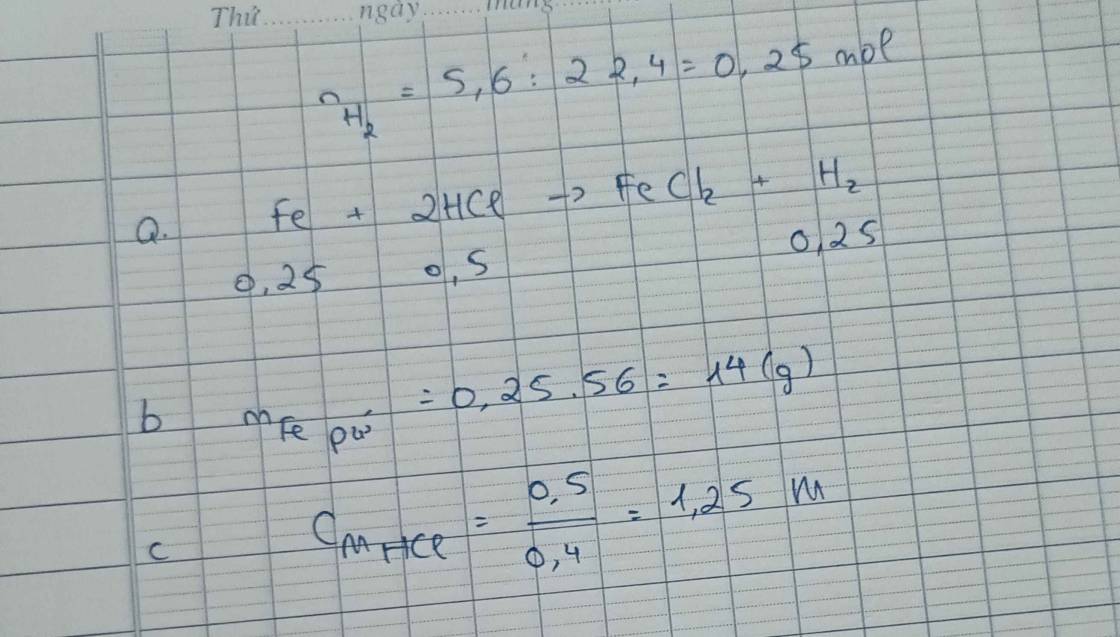

\(n_{H2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,15 0,3 0,15
\(n_{Fe}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_{Fe}=0,15.56=8,4\left(g\right)\)
\(n_{HCl}=\dfrac{0,15.2}{1}=0,3\left(mol\right)\)
50ml = 0,05l
\(C_{M_{HCl}}=\dfrac{0,3}{0,05}=6\left(M\right)\)
Chúc bạn học tốt