Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(H_2SO_4+BaCl_2\rightarrow BaSO_{4\downarrow}+2HCl\)
b, Ta có: \(m_{H_2SO_4}=114.20\%=22,8\left(g\right)\Rightarrow n_{H_2SO_4}=\dfrac{22,8}{96}=0,2375\left(mol\right)\)
\(m_{BaCl_2}=400.5,2\%=20,8\left(g\right)\Rightarrow n_{BaCl_2}=\dfrac{20,8}{208}=0,1\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,2375}{1}>\dfrac{0,1}{1}\), ta được H2SO4 dư.
Theo PT: \(n_{BaSO_4}=n_{BaCl_2}=0,1\left(mol\right)\Rightarrow m_{BaSO_4}=0,1.233=23,3\left(g\right)\)
c, Theo PT: \(\left\{{}\begin{matrix}n_{H_2SO_4\left(pư\right)}=n_{BaCl_2}=0,1\left(mol\right)\\n_{HCl}=2n_{BaCl_2}=0,2\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2SO_4\left(dư\right)}=0,2375-0,1=0,1375\left(mol\right)\)
Ta có: m dd sau pư = 114 + 400 - 23,3 = 490,7 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,1375.98}{490,7}.100\%\approx2,75\%\\C\%_{HCl}=\dfrac{0,2.36,5}{490,7}.100\%\approx1,49\%\end{matrix}\right.\)

nSO3=0,2mol
PTHH: SO3+H2O=> H2SO4
0,2--------------->0,2
=> Cm H2SO4=0,2:0,1=0,2M
b) bạn gọi x,y là lần lượt là số mon của từng chất trong B
rồi viết PTHH: từ PTHH rồi lập ra hệ pt
rồi gải x,y là xong rồi

Sửa đề cho dễ làm : dd K2CO3 13,8%
PTHH: \(2CH_3COOH+K_2CO_3\rightarrow2CH_3COOK+H_2O+CO_2\uparrow\)
a+b) Ta có: \(n_{CH_3COOH}=\dfrac{150\cdot12\%}{60}=0,3\left(mol\right)\)
\(\Rightarrow n_{K_2CO_3}=n_{CO_2}=0,15\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}m_{ddK_2CO_3}=\dfrac{0,15\cdot138}{13,8\%}=150\left(g\right)\\V_{CO_2}=0,15\cdot22,4=3,36\left(l\right)\end{matrix}\right.\)
c) Theo PTHH:: \(n_{CH_3COOK}=0,3\left(mol\right)\) \(\Rightarrow m_{CH_3COOK}=0,3\cdot98=29,4\left(g\right)\)
Mặt khác: \(m_{CO_2}=0,15\cdot44=6,6\left(g\right)\)
\(\Rightarrow m_{dd}=m_{ddCH_3COOH}+m_{ddK_2CO_3}-m_{CO_2}=293,4\left(g\right)\)
\(\Rightarrow C\%_{CH_3COOK}=\dfrac{29,4}{293,4}\cdot100\%\approx10,02\%\)

a) Đặt: nMg=x(mol); nZnO=y(mol)
nH2SO4= 0,2(mol)
PTHH: Mg + H2SO4 -> MgSO4 + H2
x___________x____x_______x(mol)
ZnO + H2SO4 -> ZnSO4 + H2O
y____y______y(mol)
Ta có:
\(\left\{{}\begin{matrix}24x+81y=12,9\\22,4x=4,48\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
mMg=0,2.24=4,8(g)
%mMg=(4,8/12,9).100=37,209%
=>%mZnO=62,791%
b) nH2SO4=x+y=0,3(mol)
=> \(C\%ddH2SO4=\dfrac{0,3.98}{120}.100=24,5\%\)

\(a.n_{H_2}=\dfrac{6,72}{22,4}=0,3mol\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,2 0,3 0,1 0,3
\(m_{Al}=0,2.27=5,4g\\ b.C_{M\left(H_2SO_4\right)}=\dfrac{0,3}{0,45}=\dfrac{2}{3}M\\ c.2H_2+O_2\underrightarrow{t^0}2H_2O\)
0,3 0,15 0,3
\(V_{O_2}=0,15.22,4=3,36l\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{Al}=\dfrac{2}{3}n_{H_2}=\dfrac{2}{3}.0,3=0,2\left(mol\right)\\ a,m_{Al}=0,2.27=5,4\left(g\right)\\ n_{H_2SO_4}=n_{H_2}=0,3\left(mol\right)\\ b,C_{MddH_2SO_4}=\dfrac{0,3}{0,45}=\dfrac{2}{3}\left(M\right)\\ 2H_2+O_2\rightarrow\left(t^o\right)2H_2O\\ n_{O_2}=\dfrac{n_{H_2}}{2}=\dfrac{0,3}{2}=0,15\left(mol\right)\\ c,V_{O_2\left(đktc\right)}=0,15.22,4=3,36\left(l\right)\)

nAl= 0,5(mol)
a) PTHH: 2 Al + 6 HCl -> 2 AlCl3 + 3 H2
nHCl= 6/2 . 0,5= 1,5(mol)
=>mHCl= 1,5.36,5=54,75(mol)
=> mddHCl= (54,75.100)/18,25=300(g)
b) nH2= 3/2. 0,5=0,75(mol)
=>V(H2,đktc)=0,75.22,4=16,8(l)
c) nAlCl3= nAl= 0,5(mol) -> mAlCl3=0,5. 133,5=66,75(g)
mddAlCl3=mAl+ mddHCl - mH2= 13,5 + 300-0,75.2=312(g)
=> \(C\%ddAlCl3=\dfrac{66,75}{312}.100\approx21,394\%\)


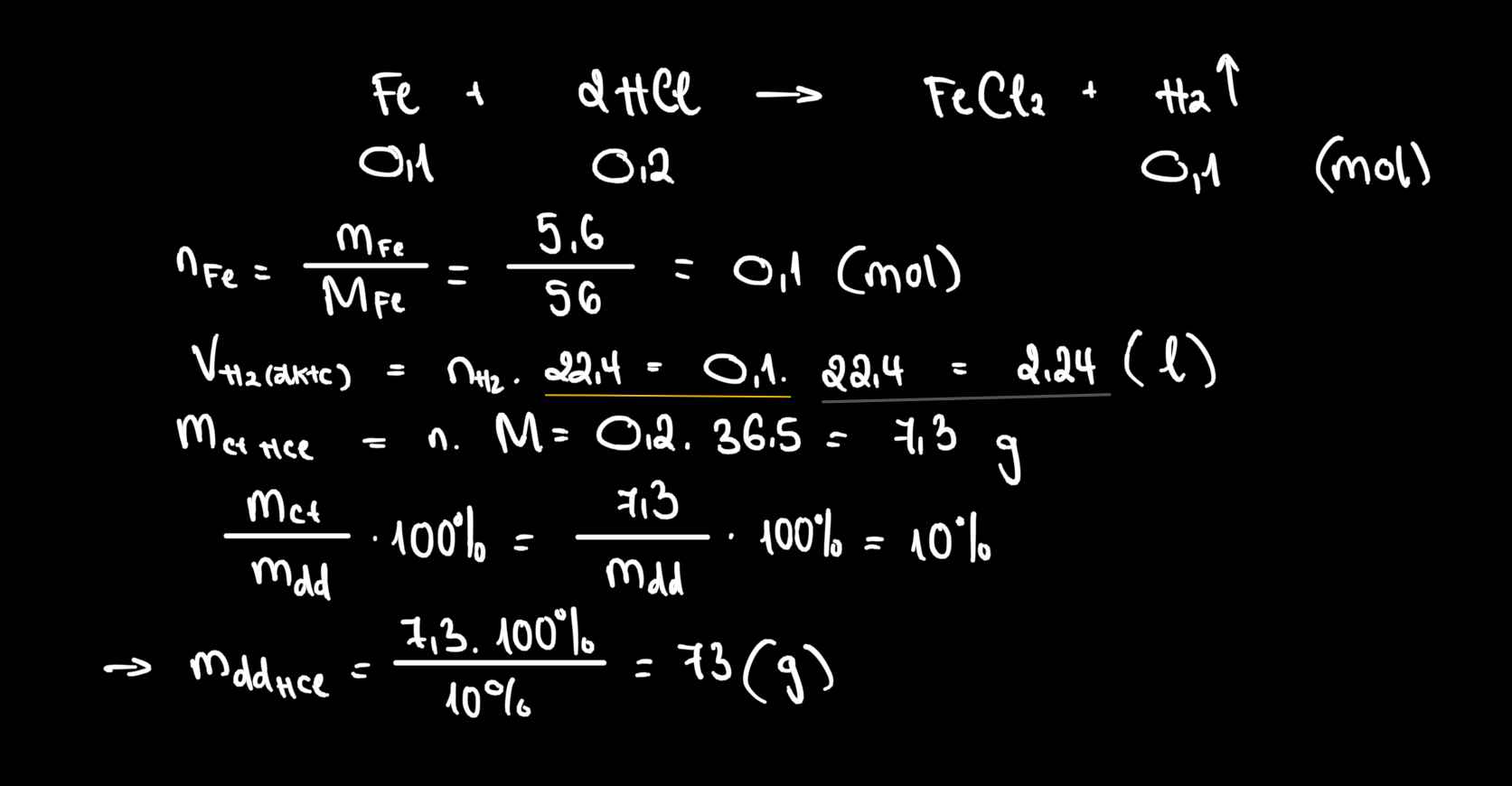
\(n_{BaSO_4}=\dfrac{58.25}{233}=0.25\left(mol\right)\)
\(BaCl_2+SO_3+H_2O\rightarrow BaSO_4+2HCl\)
\(0.25........0.25.......................0.25........0.5\)
\(V_{SO_3}=0.25\cdot22.4=5.6\left(l\right)\)
\(m_{dd_{BaCl_2}}=\dfrac{0.25\cdot208}{20\%}=260\left(g\right)\)
\(m_{dd}=m_{SO_3}+m_{dd_{BaCl_2}}-m_{BaSO_4}=0.25\cdot80+260-58.25=221.75\left(g\right)\)
\(C\%_{HCl}=\dfrac{0.5\cdot36.5}{221.75}\cdot100\%=8.2\%\)