Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(Mg+H_2SO_4\rightarrow MgSO_4+H_2\)
\(MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
b, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT: \(n_{Mg}=n_{H_2}=0,1\left(mol\right)\Rightarrow m_{Mg}=0,1.24=2,4\left(g\right)\)
\(\Rightarrow m_{MgO}=4,4-2,4=2\left(g\right)\)
c, \(n_{MgO}=\dfrac{2}{40}=0,05\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{Mg}+n_{MgO}=0,15\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,15.98=14,7\left(g\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{14,7}{19,6\%}=75\left(g\right)\)

a. PTHH:
2Al + 3H2SO4 ---> Al2(SO4)3 + 3H2
Cu + H2SO4 ---x--->
b. Theo PT: \(n_{Al}=\dfrac{2}{3}.n_{H_2}=\dfrac{2}{3}.\dfrac{6,72}{22,4}=0,2\left(mol\right)\)
=> \(m_{Al}=0,2.27=5,4\left(g\right)\)
=> \(m_{Cu}=10-5,4=4,6\left(g\right)\)
c. \(\%_{m_{Al}}=\dfrac{5,4}{10}.100\%=54\%\)
\(\%_{m_{Cu}}=100\%-54\%=46\%\)
d. Theo PT: \(n_{H_2SO_4}=n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
=> \(m_{H_2SO_4}=0,3.98=29,4\left(g\right)\)
Ta có: \(C_{\%_{H_2SO_4}}=\dfrac{29,4}{m_{dd_{H_2SO_4}}}.100\%=20\%\)
=> \(m_{dd_{H_2SO_4}}=147\left(g\right)\)

pthh: Zn+HCl→ZnCl2+H2 (1)
ZnO+HCl→ZnCl2+H2O (2)
theo bài ra số mol của H2=0,2 (mol)
theo pt1 ta có nZn=nH2=0,2 (mol)
⇒ mZn=0,2 .65=13 (g)→mZnO=21,1-13=8,1 (g) →nZnO=0,1 (mol)
%Zn=13.100%/21,1=61,61%
%ZnO=38,39%
Theo pt 1 nHCl=2nZn=0,4(mol) (3)
Theo pt2 nHCl=2nZnO=0,4 (mol) (4)
Từ 3,4 ⇒nHCl=0,8 (mol)
V HCl=0,4 (lít)=400ml

a, \(n_{CO_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
PT: \(MgO+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2O\)
\(MgCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+CO_2+H_2O\)
Theo PT: \(n_{MgCO_3}=n_{CO_2}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{MgCO_3}=\dfrac{0,1.84}{10,4}.100\%\approx80,77\%\\\%m_{MgO}\approx19,23\%\end{matrix}\right.\)
b, \(n_{MgO}=\dfrac{10,4-0,1.84}{40}=0,05\left(mol\right)\)
Theo PT: \(n_{CH_3COOH}=2n_{MgO}+2n_{MgCO_3}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{CH_3COOH}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\)

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(Ca+2HCl\rightarrow CaCl_2+H_2\)
0,1 0,1 ( mol )
\(\rightarrow\left\{{}\begin{matrix}\%m_{Ca}=\dfrac{0,1.40}{10}.100=40\%\\\%m_{MgO}=100\%-40\%=60\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}C\%_{CaCl_2}=\dfrac{0,1.111}{10+390,2-0,1.2}.100=2,775\%\\C\%_{MgO}=\dfrac{4}{10+390,2-0,1.2}.100=1\%\end{matrix}\right.\)

a)
$Fe + 2HCl \to FeCl_2 + H_2$
$FeO + 2HCl \to FeCl_2 + H_2O$
b)
Theo PTHH : $n_{Fe} = n_{H_2} = \dfrac{3,36}{22,4} = 0,15(mol)$
$m_{Fe} = 0,15.56 = 8,4(gam)$
$m_{FeO} = 12 - 8,4 = 3,6(gam)$
$n_{FeO} =0,05(mol)$
Theo PTHH : $n_{HCl} = 2n_{Fe} + 2n_{FeO} = 0,4(mol)$
$V_{dd\ HCl} = \dfrac{0,4}{2} = 0,2(lít)$
c) $Fe + CuSO_4 \to FeSO_4 + Cu$
$n_{Cu} = n_{Fe} = 0,15(mol) \Rightarrow m_{chất\ rắn} = m_{FeO} + m_{Cu}$
$= 3,6 + 0,15.64 = 13,2(gam)$

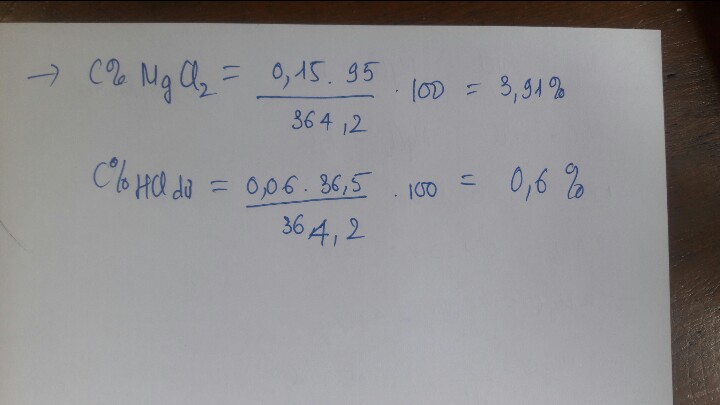
a/ \(n_{CO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(MgCO_3+H_2SO_4\rightarrow MgSO_4+H_2O+CO_2\)
\(0,5---0,5----0,5---0,5-0,5\)
\(MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
\(b---b----b-----b\)
\(\Rightarrow m_{MgCO_3}=0,5.\left(24+12+16.3\right)=42\left(g\right)\)
\(\dfrac{m_{MgCO_3}}{m_{MgO}}=\dfrac{7}{3}\Rightarrow m_{MgO}=42.\dfrac{3}{7}=18\left(g\right)\Rightarrow n_{MgO}=b=0,45\left(mol\right)\)
\(\Rightarrow n_{H_2SO_4}=0,45+0,5=0,95\left(mol\right)\) \(\Rightarrow m_{dd}=\dfrac{0,95.98}{0,05}=1862\left(g\right)\)