Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi mol của Mg và Al là x, y mol
=> 24x + 27y = 12,6 (1)
nH2 = 0,6 mol => x + 1,5y = 0,6 (2)
Từ (1) (2) => x = 0,3 ; y = 0,2
=> %Mg = 57,14%
=> %Al = 42,86%

PTHH
Mg + 2HCl ----> MgCl2 + H2 (1)
MgO + 2HCl -----> MgCl2 + H2O (2)
a) Theo pt(1) n Mg = n H2 = \(\frac{1,12}{22,4}\) = 0,05 (mol)
==> m Mg = 0,005 . 24=1,2 (g)
%m Mg = \(\frac{1,2}{3,2}\). 100%= 37,5%
%m MgO= 100% - 37,5%= 62,5%
b)m dd sau pư = 3,2 + 246,9 - 0,05 . 2=250 (g)
Theo pt(1)(2) n MgCl2(1) = n Mg = 0,05 mol
n MgCl2 (2) = n MgO=\(\frac{3,2-1,2}{40}\)=0,05(mol)
==> tổng n MgCl2 = 0,1 (mol) ---->m MgCl2 = 9,5 (g)
C%(MgCl2)= \(\frac{9,5}{250}\) .100% = 3,8%

a, Ta có: 65nZn + 27nAl = 11,9 (1)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Theo PT: \(n_{H_2}=n_{Zn}+\dfrac{3}{2}n_{Al}=\dfrac{9,916}{24,79}=0,4\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{Zn}=0,1\left(mol\right)\\n_{Al}=0,2\left(mol\right)\end{matrix}\right.\)
⇒ mZn = 0,1.65 = 6,5 (g)
mAl = 0,2.27 = 5,4 (g)
b, Theo PT: nZnCl2 = nZn = 0,1 (mol)
nAlCl3 = nAl = 0,2 (mol)
⇒ m muối = 0,1.136 + 0,2.133,5 = 40,3 (g)
c, Theo PT: nHCl = 2nH2 = 0,8 (mol)
\(\Rightarrow m_{ddHCl}=\dfrac{0,8.36,5}{10\%}=292\left(g\right)\)

\(A.Mg+H_2SO_4\rightarrow MgSO_4+H_2\\ B.n_{H_2}=\dfrac{1,12}{22,4}=0,05mol\\ Mg+H_2SO_4\rightarrow MgSO_4+H_2\)
0,05 0,05 0,05 0,05
\(\%m_{Mg}=\dfrac{0,05.24}{6,4}\cdot100=18,75\%\\ \%m_{Cu}=100-18,75=81,25\%\\ C.m_{ddH_2SO_4}=\dfrac{0,05.98}{20}\cdot100=24,5g\)
a) Pt : \(Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\)
Theo Pt : \(n_{Mg}=n_{H2SO4}=n_{MgSO4}=n_{H2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
b) \(\%m_{Mg}=\dfrac{0,05.24}{6,4}.100\%=18,75\%\)
\(\%m_{Cu}=100\%-18,75\%=81,25\%\)
c) \(m_{H2SO4}=0,05.98=4,9\left(g\right)\)
\(\Rightarrow m_{ddH2SO4}=\dfrac{4.100\%}{20\%}=20\left(g\right)\)
Chúc bạn học tốt

a) Gọi \(\left\{{}\begin{matrix}n_{Mg}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\)
PTHH: Mg + H2SO4 --> MgSO4 + H2
a--->a---------->a-------->a
Fe + H2SO4 --> FeSO4 + H2
b--->b----------->b------>b
=> \(m_{H_2SO_4}=98a+98b\left(g\right)\)
=> \(m_{ddH_2SO_4}=\dfrac{\left(98a+98b\right).100}{19,6}=500a+500b\left(g\right)\)
mdd sau pư = 24a + 56b + 500a + 500b - 2a - 2b = 522a + 554b (g)
Có: \(C\%_{FeSO_4}=\dfrac{152b}{522a+554b}.100\%=7,17\%\)
=> a = 3b
\(C\%_{MgSO_4}=\dfrac{120a}{522a+554b}.100\%=16,98\%\)
b)
Có: \(\left\{{}\begin{matrix}a=3b\\24a+56b=1,92\end{matrix}\right.\)
=> a = 0,045; b = 0,015
\(n_{CuSO_4}=0,1.1=0,1\left(mol\right)\)
PTHH: Mg + CuSO4 --> MgSO4 + Cu
0,045->0,045----->0,045
Fe + CuSO4 --> FeSO4 + Cu
0,015-->0,015----->0,015
=> \(\left\{{}\begin{matrix}n_{CuSO_4\left(dư\right)}=0,04\left(mol\right)\\n_{MgSO_4}=0,045\left(mol\right)\\n_{FeSO_4}=0,015\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(CuSO_4\left(dư\right)\right)}=\dfrac{0,04}{0,1}=0,4M\\C_{M\left(MgSO_4\right)}=\dfrac{0,045}{0,1}=0,45M\\C_{M\left(FeSO_4\right)}=\dfrac{0,015}{0,1}=0,15M\end{matrix}\right.\)

a)
$RCO_3 + H_2SO_4 \to RSO_4 + CO_2 + H_2O$
Theo PTHH :
$n_{RCO_3} = n_{RSO_4}$
Suy ra : \(\dfrac{12,4}{R+60}=\dfrac{16}{R+96}\)
Suy ra : R = 64(Cu)
Vậy muối là $CuCO_3$
b)
$n_{CO_2} = n_{H_2SO_4} = n_{CuSO_4} = 16 : 160 = 0,1(mol)$
$m_{dd\ H_2SO_4} = \dfrac{0,1.98}{9,8\%} = 100(gam)$
$m_{dd\ sau\ pư} = 12,4 + 100 -0,1.44 = 108(gam)$
$C\%_{CuSO_4} = \dfrac{16}{108}.100\% = 14,81\%$
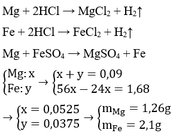
$m_{H_2SO_4} = a.C\%(gam) \Rightarrow n_{H_2SO_4} = \dfrac{a.C\%}{98}$
$m_{H_2O\ trong\ dd\ axit} = a - a.C\% \Rightarrow n_{H_2O} = \dfrac{a - a.C\%}{18}$
$2Na + H_2SO_4 \to Na_2SO_4 + H_2$
$Mg + H_2SO_4 \to MgSO_4 + H_2$
$2Na + 2H_2O \to 2NaOH + H_2$
Theo PTHH :
$n_{H_2} = n_{H_2SO_4} + \dfrac{1}{2}n_{H_2O}$
$\Rightarrow \dfrac{0,05a}{2} = \dfrac{a.C\%}{98} + \dfrac{1}{2}.\dfrac{a - a.C\%}{18}$
$\Rightarrow C\% = 0,158 = 15,8\%$