Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) 2Al + 6HCl -> 2AlCl3 + 3H2
Al2O3 + 6HCl -> 2AlCl3 + 3H2O
nH2 = 0,15mol => nAl=0,1mol => mAl=2,7g; mAl2O3 = 10,2g => nAl2O3 = 0,1mol
=>%mAl=20,93% =>%mAl2O3 = 79,07%
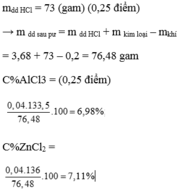
b) nHCl = 0,1.3+0,1.6=0,9 mol=>mHCl(dd)=100g
mddY=12,9+100-0,15.2=112,6g
mAlCl3=22,5g=>C%=19,98%

a)\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PTHH: 2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Mol: x 1,5x
PTHH: Mg + H2SO4 → MgSO4 + H2
Mol: y y
Ta có: \(\left\{{}\begin{matrix}27x+24y=5,1\\1,5x+y=0,25\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,1\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{0,1.27.100\%}{5,1}=52,94\%;\%m_{Mg}=100-52,94=47,06\%\)
b)
PTHH: 2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Mol: 0,1 0,15 0,05
PTHH: Mg + H2SO4 → MgSO4 + H2
Mol: 0,1 0,1 0,1
\(m_{ddH_2SO_4}=\dfrac{\left(0,1+0,15\right).98.100}{9,8}=250\left(g\right)\)
mdd sau pứ = 5,1+250-0,15.2 = 254,8(g)
\(C\%_{ddAl_2\left(SO_4\right)_3}=\dfrac{0,05.342.100\%}{254,8}=6,71\%\)
\(C\%_{ddMgSO_4}=\dfrac{0,1.120.100\%}{254,8}=4,71\%\)

a, PT: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
Ta có: \(n_{H_2}=\dfrac{0,672}{22,4}=0,03\left(mol\right)\)
Theo PT: \(n_{Mg}=n_{H_2}=0,03\left(mol\right)\)
\(\Rightarrow m_{Mg}=0,03.24=0,72\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,72}{1,74}.100\%\approx41,38\%\\\%m_{AlCl_3}\approx58,62\%\end{matrix}\right.\)
b, Theo PT: \(n_{HCl}=2n_{H_2}=0,06\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,06.36,5=2,19\left(g\right)\)
\(\Rightarrow C\%_{ddHCl}=\dfrac{2,19}{500}.100\%=0,438\%\)
Bạn tham khảo nhé!
a, nH2 = 0,03 ( mol )
=> nMg = nH2 = 0,03 ( mol )
=> mMg = 0,72 g
=> %Mg \(\approx\) 41,38 % .
=> % Al \(\approx\) 58,62 % .
b, Có : nH2 = 0,03 mol
=> nHCl = nHCltừ Al2O3 + nHCltừ Mg = 0,06 + 0,06 = 0,12 ( mol )
=> mHCl = 4,38 ( g )
Lại có : mdd = mhh + mddHCl = 501,74 ( g )
=> \(C\%=\dfrac{m_{HCl}}{m_{dd}}.100\%\approx0,87\%\)
( chắc đoạn trên là Al2O3 :vvvv )

a)
Gọi $n_{Zn} = a(mol) ; n_{Al} = b(mol) \Rightarrow 65a + 27b = 11,9(1)$
$Zn + 2HCl \to ZnCl_2 + H_2$
$2Al + 6HCl \to 2AlCl_3 + 3H_2$
Theo PTHH :
$n_{H_2} = a + 1,5b = \dfrac{8,96}{22,4} = 0,4(2)$
Từ (1)(2) suy ra : a = 0,1; b = 0,2
$m_{Zn} = 0,1.65 = 6,5(gam)$
$m_{Al} = 0,2.27 = 5,4(gam)$
b) $n_{HCl} = 2n_{H_2} = 0,8(mol)$
$C\%_{HCl} = \dfrac{0,8.36,5}{125}.100\% = 23,36\%$

a)
Gọi số mol Mg, Al là a, b (mol)
=> 24a + 27b = 26,25 (1)
\(n_{H_2}=\dfrac{30,8}{22,4}=1,375\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
a-->2a--------->a------>a
2Al + 6HCl --> 2AlCl3 + 3H2
b---->3b------->b------>1,5b
=> a + 1,5b = 1,375 (2)
(1)(2) => a = 0,25 (mol); b = 0,75 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,25.24}{26,25}.100\%=22,857\%\\\%m_{Al}=\dfrac{0,75.27}{26,25}.100\%=77,143\%\end{matrix}\right.\)
b)
nHCl = 2a + 3b = 2,75 (mol)
=> mHCl = 2,75.36,5 = 100,375 (g)
=> \(m_{dd.HCl}=\dfrac{100,375.100}{10}=1003,75\left(g\right)\)
c)
mdd sau pư = 1003,75 + 26,25 - 1,375.2 = 1027,25 (g)
\(\left\{{}\begin{matrix}C\%_{MgCl_2}=\dfrac{0,25.95}{1027,25}.100\%=2,312\%\\C\%_{AlCl_3}=\dfrac{0,75.133,5}{1027,25}.100\%=9,747\%\end{matrix}\right.\)

Mg+2HCl->MgCl2+H2
x------2x--------x---------x
2Al+6HCl->2AlCl3+3H2
y---------3y-----y--------3\2y
ta có :
\(\left\{{}\begin{matrix}24x+27y=11,7\\x+\dfrac{3}{2}y=0,6\end{matrix}\right.\)
=>x=0,15 mol, y=0,3 mol
=>%mMg=\(\dfrac{0,15.24}{11,7}.100=30,77\%\)
=>%mAl=100-30,77=69,23%
b)
m HCl=1,2.36,5=43,8g
=>C%=\(\dfrac{43,8}{200}.100\)=21,9%