Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(C_{M_{HCl}}=a\left(M\right),C_{M_{H_2SO_4}}=b\left(M\right)\)
\(n_{HCl}=a\left(mol\right),n_{H_2SO_4}=b\left(mol\right)\)
\(n_{NaOH}=0.4\cdot0.5=0.2\left(mol\right)\)
\(NaOH+HCl\rightarrow NaCl+H_2O\)
\(a..........a.........a\)
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
\(2b............b..........b\)
\(n_{NaOH}=a+2b=0.2\left(mol\right)\left(1\right)\)
\(m_{muối}=58.5a+142b=12.95\left(g\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.1,b=0.05\)
\(\left[H^+\right]=0.1+0.05\cdot2=0.2\left(M\right)\)
\(\left[Cl^-\right]=0.1\left(M\right)\)
\(\left[SO_4^{2-}\right]=0.05\left(M\right)\)
\(b.\)
\(pH=-log\left(0.2\right)=0.7\)

`n_{BaCl_2}=200.10^{-3}.1=0,2(mol)`
`n_{KCl}=100.10^{-3}.2=0,2(mol)`
`->n_{Cl^-}=2n_{BaCl_2}+n_{KCl}=0,6(mol)`
`->[Cl^-]={0,6}/{(200+100).10^{-3}}=2M`

a, \(n_{Ba\left(OH\right)_2}=0,1.0,1=0,01\left(mol\right)=n_{Ba^{2+}}\)
\(\Rightarrow n_{OH^-}=2n_{Ba\left(OH\right)_2}=0,02\left(mol\right)\)
\(n_{NaOH}=0,1.0,1=0,01\left(mol\right)=n_{Na^+}=n_{OH^-}\)
⇒ ΣnOH- = 0,02 + 0,01 = 0,03 (mol)
\(n_{H_2SO_4}=0,4.0,0175=0,007\left(mol\right)=n_{SO_4^{2-}}\)
\(\Rightarrow n_{H^+}=2n_{H_2SO_4}=0,014\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
0,014___0,014 (mol) ⇒ nOH- dư = 0,03 - 0,014 = 0,016 (mol)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\)
0,007____0,007_____0,007 (mol) ⇒ nBa2+ dư = 0,01 - 0,007 = 0,003 (mol)
⇒ m = 0,007.233 = 1,631 (g)
\(\left[OH^-\right]=\dfrac{0,016}{0,1+0,4}=0,032\left(M\right)\)
\(\left[Ba^{2+}\right]=\dfrac{0,003}{0,1+0,4}=0,006\left(M\right)\)
\(\left[Na^+\right]=\dfrac{0,01}{0,1+0,4}=0,02\left(M\right)\)
b, pH = 14 - (-log[OH-]) ≃ 12,505
\(n_{Ba^{2+}}=0,1.0,1=0,01\left(mol\right)\)
\(n_{SO_4^{2-}}=0,4.0,0175=7.10 ^{-3}\left(mol\right)\)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\downarrow\)
\(\Rightarrow m=m_{BaSO_4}=7.10^{-3}.233=1,631\left(g\right)\)
Ta có:
\(n_{H^+}=0,4.0,0175.2=0,014\left(mol\right)\)
\(n_{OH^-}=0,1.0,1.2+0,1.0,1=0,03\left(mol\right)\)
Trong dung dịch X:
\(n_{OH^-}=0,03-0,014=0,016\left(mol\right)\)\(\Rightarrow\left[OH^-\right]=\dfrac{0,016}{0,1+0,4}=0,032\left(M\right)\)
\(n_{Ba^{2+}}=0,01-7.10^{-3}=3.10^{-3}\left(mol\right)\Rightarrow\left[Ba^{2+}\right]=\dfrac{3.10^{-3}}{0,1+0,4}=6.10^{-3}\left(M\right)\)
\(n_{Na^+}=0,1.0,1=0,01\left(mol\right)\Rightarrow\left[Na^+\right]=0,02\)
\(pOH=-lg\left(0,032\right)\approx1,5\Rightarrow pH=14-1,5=12,5\)
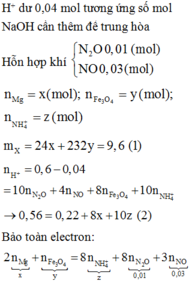
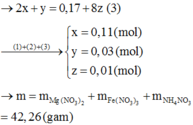

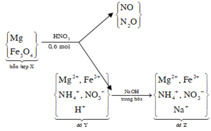

$n_{Na^+} = 0,2.2 + 0,1.2 = 0,6(mol)$
$n_{Cl^-} = 0,2.2 + 0,1.1,5 = 0,55(mol)$
$n_{NO_3^-} = 0,2.1 = 0,2(mol)$
$n_{OH^-} = 0,1.2 = 0,2(mol)$
$n_{K^+} = 0,2.1 + 0,1.1,5 = 0,35(mol)$
$V_{dd} = 0,2 + 0,1 = 0,3(lít)$
Suy ra:
$[Na^+] = \dfrac{0,6}{0,3} = 2M$
$[Cl^-] = \dfrac{0,55}{0,3}= 1,83M$
$[OH^-] = [NO_3^-] = \dfrac{0,2}{0,3} = 0,67M$
$[K^+] = \dfrac{0,35}{0,3} = 1,167M$
$m_{muối} = m_{NaCl} + m_{KNO_3} + m_{KCl} = 0,2.2.58,5 + 0,2.101 + 0,1.1,5.74,5$
$= 54,775(gam)$