Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
Gọi số mol Fe, Al, Ag trong mỗi phần là a, b,c (mol)
=> 56a + 27b + 108c = 5,19 (1)
Phần 1:
\(n_{H_2}=\dfrac{2,352}{22,4}=0,105\left(mol\right)\)
PTHH: Fe + H2SO4 --> FeSO4 + H2
a----->a------------------>a
2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
b------>1,5b------------------->1,5b
=> a + 1,5b = 0,105 (2)
Phần 2:
\(n_{SO_2}=\dfrac{2,912}{22,4}=0,13\left(mol\right)\)
PTHH: 2Al + 6H2SO4 --> Al2(SO4)3 + 3SO2 + 6H2O
b----->3b-------------------->1,5b
2Fe + 6H2SO4 --> Fe2(SO4)3 + 3SO2 + 6H2O
a------>3a--------------------->1,5a
2Ag + 2H2SO4 --> Ag2SO4 + SO2 + 2H2O
c-------->c------------------>0,5c
=> 1,5a + 1,5b + 0,5c = 0,13 (3)
(1)(2)(3) => a = 0,03 (mol); b = 0,05 (mol); c = 0,02 (mol)
=> \(\left\{{}\begin{matrix}m_{Fe}=2.0,03.56=3,36\left(g\right)\\m_{Al}=2.0,05.27=2,7\left(g\right)\\m_{Ag}=2.0,02.108=4,32\left(g\right)\end{matrix}\right.\)
b)
- Phần 1:
\(n_{H_2SO_4}=a+1,5b=0,105\left(mol\right)\)
- Phần 2:
\(n_{H_2SO_4}=3a+3b+c=0,26\left(mol\right)\)

\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\)
\(FeCl_2+2NaOH\rightarrow Fe\left(OH\right)_2+2NaCl\)
\(NaOH+Al\left(OH\right)_3\rightarrow NaAlO_2+2H_2O\)
\(4Fe\left(OH\right)_2+O_2\underrightarrow{^{^{t^0}}}2Fe_2O_3+4H_2O\)
Ta có :
\(n_{Fe_2O_3}=\dfrac{16}{160}=0.1\left(mol\right)\)
Dựa vào PTHH ta thấy :
\(n_{Fe}=2\cdot n_{Fe_2O_3}=2\cdot0.1=0.2\left(mol\right)\)
\(m_{Fe}=0.2\cdot56=11.2\left(g\right)\)
\(\Rightarrow m_{Al}=19.3-11.2=8.1\left(g\right)\)
\(\%Al=\dfrac{8.1}{19.3}\cdot100\%=41.96\%\)

mk viết nhầm. HSO là H2SO4. BaCl là BaCl2.
NaCO là Na2CO3 . CO là khí CO2

a) \(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
Theo PTHH ta có: \(n_{MgCl_2}=n_{Mg}=0,1\left(mol\right)\)
\(\Rightarrow m_{MgCl_2}=n_{MgCl_2}.M_{MgCl_2}=0,1.95=9,5\left(g\right)\)
b) Theo PTHH ta có: \(n_{H_2}=n_{Mg}=0,1\left(mol\right)\)
\(\Rightarrow V_{H_2}=n_{H_2}.22,4=0,1.22,4=2,24\left(l\right)\)
c) \(2H_2+O_2\rightarrow2H_2O\)
Theo PTHH: \(n_{H_2O}=\dfrac{0,1.2}{2}=0,1\left(mol\right)\)
\(\Rightarrow m_{H_2O}=n_{H_2O}.M_{H_2O}=0,1.18=1,8\left(g\right)\)
a)mMg= 2,4/24=0,1(mol) nMgCl2=0,1.1/1=0,1 (mol) mMgCl2= 0,1.95=9,5(g) b)nH2=0,1.1/1=0,1(mol) v=n.22,4=2,24(lít

Phần 1 :
$m_{Cu} = 0,4(gam)$
Gọi $n_{Fe} = a ; n_{Al} = b \Rightarrow 56a + 27b + 0,4 = 1,5 : 2 = 0,75(1)$
$Fe + 2HCl \to FeCl_2 + H_2$
$2Al + 6HCl \to 2AlCl_3 + 3H_2$
$n_{H_2} = a + 1,5b = \dfrac{896}{1000.22,4} = 0,04(2)$
Từ (1)(2) suy ra a = -0,025 < 0$
$\to$ Sai đề
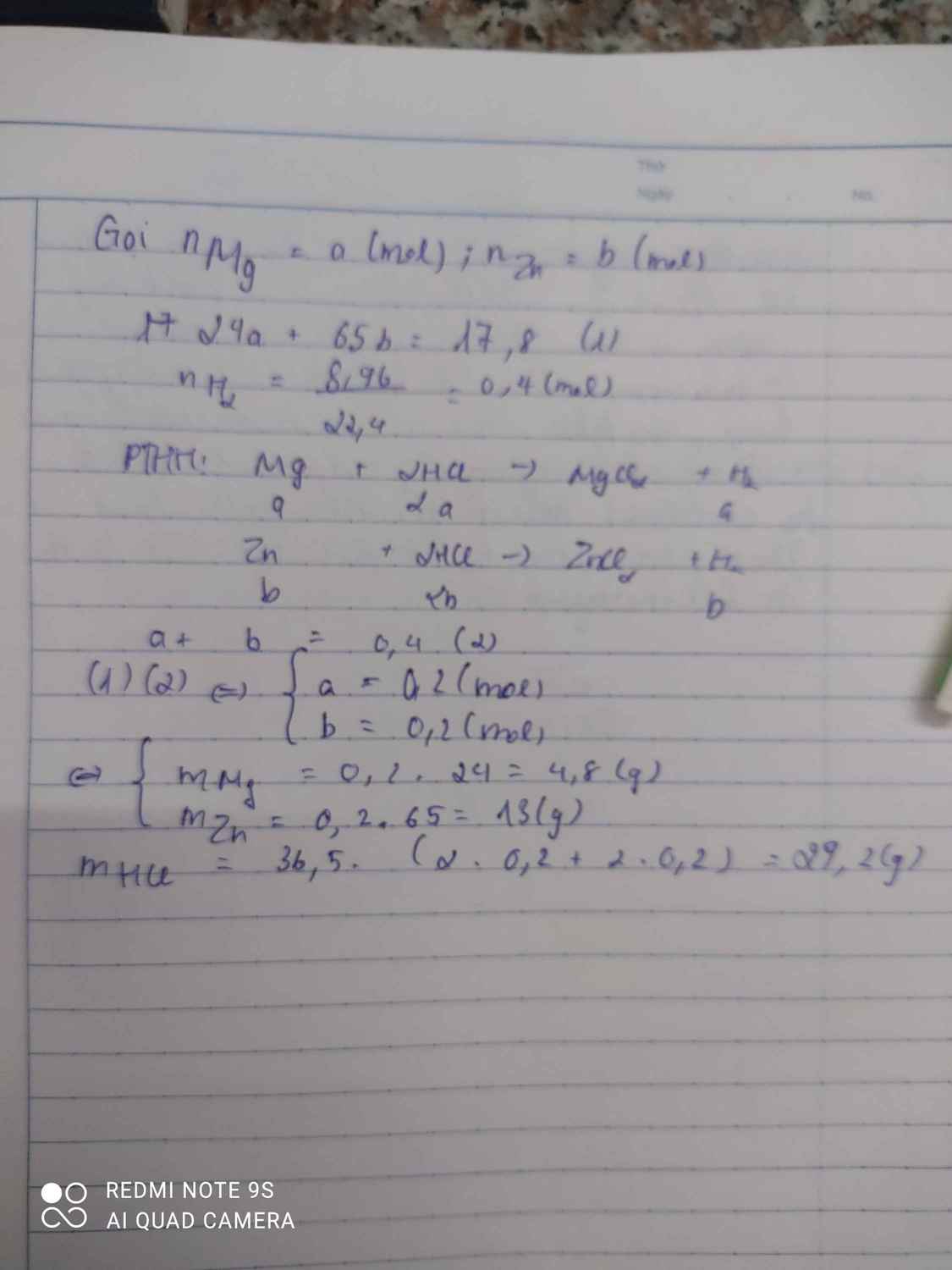
a)
\(2K+2H_2O\rightarrow2KOH+H_2\)
\(2K+2HCl\rightarrow2KCl+H_2\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(4K+O_2\underrightarrow{t^o}2K_2O\)
\(2Mg+O_2\underrightarrow{t^o}2MgO\)
b)
P1: \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: 2K + 2H2O --> 2KOH + H2
0,3<--------------------0,15
=> nK(X) = 0,3.3 = 0,9 (mol)
P2:
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: 2K + 2HCl --> 2KCl + H2
0,3------------------->0,15
Mg + 2HCl --> MgCl2 + H2
0,25<-------------------0,25
=> nMg(X) = 0,25.3 = 0,75 (mol)
P3:
PTHH: 4K + O2 --to--> 2K2O
0,3------------->0,15
2Mg + O2 --to--> 2MgO
0,25--------------->0,25
=> mAg(P3) = 34,9 - 0,15.94 - 0,25.40 = 10,8 (g)
=> mAg(X) = 10,8.3 = 32,4 (g)
a = mX = 0,9.39 + 0,75.24 + 32,4 = 85,5 (g)