Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\)
PTHH: Mg + 2HCl ---> MgCl2 + H2
0,15--------------->0,15---->0,15
CuO + H2 --to--> Cu + H2O
0,15------->0,15
=> \(\left\{{}\begin{matrix}m_{MgCl_2}=0,15.95=14,25\left(g\right)\\V_{H_2}=0,15.24,79=3,7195\left(l\right)\\m_{Cu}=0,15.64=9,6\left(g\right)\end{matrix}\right.\)
\(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\\
pthh:Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
0,15 0,15 0,15
\(m_{MgCl_2}=0,15.95=14,25\left(g\right)\\
V_{H_2}=0,3.22,4=3,36\left(L\right)\\
pthh:CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
0,3 0,3 0,3
\(m_{Cu}=0,3.64=19,2\left(g\right)\)

\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\)
PTHH: Mg + 2HCl ---> MgCl2 + H2
0,2 0,2
2H2 + O2 --to--> 2H2O
0,2 0,2
\(\rightarrow\left\{{}\begin{matrix}V_{H_2}=0,2.22,4=4,48\left(l\right)\\m_{H_2O}=0,2.18.\left(100\%-5\%\right)=3,42\left(g\right)\end{matrix}\right.\)
\(n_{Mg}=\dfrac{4,8}{24}=0,2mol\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
0,2 0,2
\(V_{H_2}=0,2\cdot22,4=4,48l\)
\(2H_2+O_2\underrightarrow{t^o}2H_2O\)
0,2 0,2
\(m_{H_2O}=0,2\cdot18\cdot\left(100-5\right)\%=3,42g\)

$a)Zn + 2HCl \to ZnCl_2 + H_2$
b)
Theo PTHH :
n H2 = n Zn = 1,3/65 = 0,02(mol)
V H2 = 0,02.22,4 = 0,448(lít)
c) $CuO + H_2 \xrightarrow{t^o} Cu + H_2O$
Ta thấy :
n CuO = 2,4/80 = 0,03 > n H2 = 0,02 nên CuO dư
Theo PTHH :
n CuO pư = n Cu = n H2 = 0,02(mol)
Sau phản ứng có :
m Cu = 0,02.64 = 1,28(gam)
m CuO dư = 2,4 - 0,02.80 = 0,8(gam)
Ta có: \(n_{Zn}=\dfrac{1,3}{65}=0,02\left(mol\right)\)
a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
_____0,02_________________0,02 (mol)
b, VH2 = 0,02.22,4 = 0,448 (l)
c, Ta có: \(n_{CuO}=\dfrac{2,4}{80}=0,03\left(mol\right)\)
PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Xét tỉ lệ: \(\dfrac{0,03}{1}>\dfrac{0,02}{1}\), ta được CuO dư.
Theo PT: \(n_{Cu}=n_{CuO\left(pư\right)}=n_{H_2}=0,02\left(mol\right)\)
⇒ nCuO (dư) = 0,01 (mol)
\(\Rightarrow m_{CuO\left(dư\right)}=0,01.80=0,89\left(g\right)\)
\(m_{Cu}=0,02.64=1,28\left(g\right)\)
Bạn tham khảo nhé!

\(n_{Zn}=\dfrac{3,25}{65}=0,05\left(mol\right)\)
PTHH: Zn + 2HCl ---> ZnCl2 + H2
0,05-->0,1------>0,05--->0,05
FeO + H2 --to--> Fe + H2O
0,05------>0,05
=> \(\left\{{}\begin{matrix}m_{ZnCl_2}=0,05.136=6,8\left(g\right)\\V_{H_2}=0,05.24,79=1,2395\left(l\right)\\m_{Fe}=0,05.56=2,8\left(g\right)\end{matrix}\right.\)
\(n_{Zn}=\dfrac{3,25}{65}=0,05\left(mol\right)\\
pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,05 0,05 0,05
\(m_{ZnCl_2}=0,05.136=6,8\left(g\right)\\
V_{H_2}=0,05.24,79=1,2395l\)
\(FeO+H_2\underrightarrow{t^o}Fe+H_2O\)
0,05 0,05 0,05
\(m_{Fe}=0,05.56=2,8g\)

a, \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
b, \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(n_{Fe_3O_4}=\dfrac{18,56}{232}=0,08\left(mol\right)\)
PT: \(Fe_3O_4+4H_2\underrightarrow{t^o}3Fe+4H_2O\)
Xét tỉ lệ: \(\dfrac{0,08}{1}>\dfrac{0,2}{4}\), ta được Fe3O4 dư.
Theo PT: \(n_{Fe}=\dfrac{3}{4}n_{H_2}=0,15\left(mol\right)\Rightarrow m_{Fe}=0,15.56=8,4\left(g\right)\)

\(a) Mg + 2HCl \to MgCl_2 + H_2\\ b) n_{MgCl_2} = n_{Mg} = \dfrac{0,24}{24} = 0,01(mol)\\ m_{MgCl_2} = 0,01.95 = 0,95(gam)\\ c) n_{H_2} = n_{Mg} = 0,01(mol) \Rightarrow V_{H_2} = 0,01.22,4 = 0,224(lít)\)
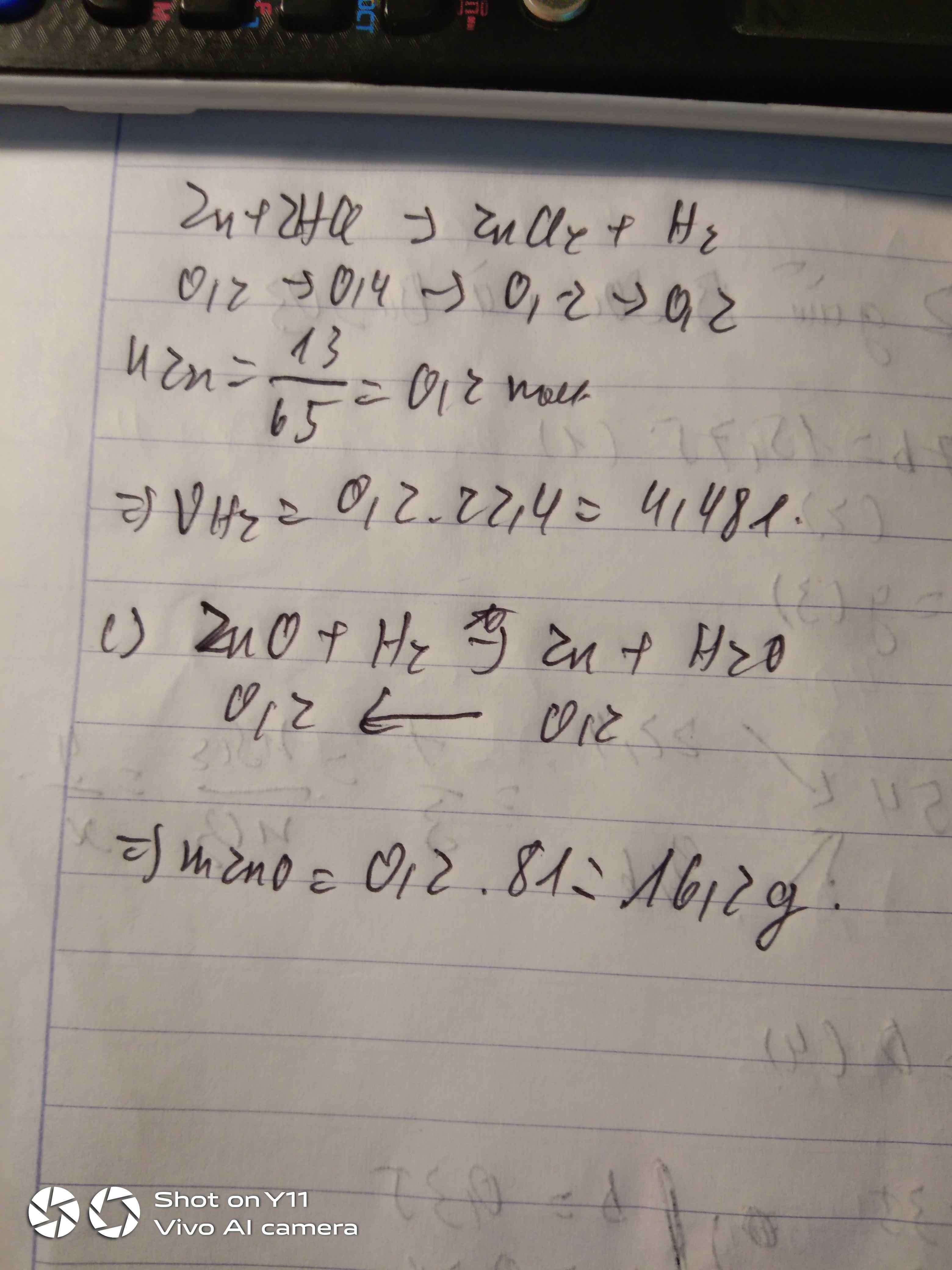

`a)PTHH:`
`Mg + 2HCl -> MgCl_2 + H_2`
`0,2` `0,2` `(mol)`
`n_[Mg]=[4,8]/24=0,2(mol)`
`b)V_[H_2]=0,2.22,4=4,48(l)`
\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\\ pthh:Mg+2HCl\rightarrow MgCl_2+H_2\)
0,2 0,2
\(V_{H_2}=0,2.22,4=4,48l\)