Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{3,36}{22,4}=0,15(mol)\\ Zn+H_2SO_4\to ZnSO_4+H_2\\ \Rightarrow n_{Zn}=0,15(mol)\\ \Rightarrow \%_{Zn}=\dfrac{0,15.65}{15,75}.100\%=61,9\%\\ \Rightarrow \%_{Cu}=100\%-61,9\%=38,1\%\\ b,n_{ZnSO_4}=0,15(mol)\\ \Rightarrow m_{ZnSO_4}=0,15.161=24,15(g)\)

\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: Fe + H2SO4 --> FeSO4 + H2
_____0,15<--------------0,15<---0,15
=> mFe = 0,15.56 = 8,4 (g)
=> mCu = 11,6 - 8,4 = 3,2 (g)
\(\left\{{}\begin{matrix}\%Fe=\dfrac{8,4}{11,6}.100\%=72,414\%\\\%Cu=\dfrac{3,2}{11,6}.100\%=27,586\%\end{matrix}\right.\)
mFeSO4 = 0,15.152 = 22,8 (g)

a)
\(n_{H_2}=\dfrac{9,916}{24,79}=0,4\left(mol\right)\)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
_____0,4<---0,4<--------0,4<----0,4
=> mZn = 0,4.65 = 26 (g)
=> \(\left\{{}\begin{matrix}\%Zn=\dfrac{26}{51,6}.100\%=50,388\%\\\%Cu=\dfrac{51,6-26}{51,6}.100\%=49,612\text{%}\end{matrix}\right.\)
b)
mZnSO4 = 0,4.161 = 64,4 (g)
c)
\(V_{ddH_2SO_4}=\dfrac{0,4}{2}=0,2\left(l\right)\)

a) \(n_{Zn}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
_____0,15<-------------0,15<---0,15
=> mZn = 0,15.65 = 9,75(g)
=> \(\left\{{}\begin{matrix}\%Zn=\dfrac{9,75}{17,75}.100\%=54,93\%\\\%Cu=100\%-54,93\%=45,07\%\end{matrix}\right.\)
b) mZnSO4 = 0,15.161=24,15(g)

Cu ko phản ứng với H2SO4 loãng
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(\Rightarrow m_{Zn}=0,1.65=6,5\left(g\right)\Rightarrow m_{Cu}=4\left(g\right)\)
\(\Rightarrow\%Zn=\dfrac{6,5}{10,5}=62\%;\%Cu=100\%-62\%=28\%\)

a) \(Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2\)
Cu không pư H2SO4 loãng
b)
\(n_{H_2}=\dfrac{2,24}{22,4}= 0,1 mol\)
Theo PTHH:
\(n_{Zn}= n_{H_2}= 0,1 mol\)
\(\Rightarrow m_{Zn}= 0,1 . 65= 6,5 g\)
\(\Rightarrow m_{Cu}= m_{hh KL} - m_{Zn}= 10 - 6,5 = 3,5 g\)
Gọi \(n_{Cu}=x\left(mol\right)\)\(;n_{Zn}=y\left(mol\right)\)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,1
\(m_{Zn}=0,1\cdot65=6,5g\)
\(m_{Cu}=10-6,4=3,6g\)
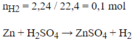
\(33,6(l) \to 3,36(l)\\ Zn+H_2SO_4 \to ZnSO_4+H_2\\ n_{H_2}=0,15(mol)\\ n_{Zn}=n_{H_2}=0,15(mol)\\ a/\\ \%m_{Zn}=\frac{0,15.65}{15,75}.100\%=61,9\%\\ \%m_{Cu}=38,06\%\\ b/\\ n_{ZnSO_4}=n_{H_2}=0,15(mol)\\ m_{ZnSO_4}=0,15.161=24,15(g)\)