Đốt cháy hoàn toàn một lượng magie thu được 2,4g MgO a) viết phương trình phản ứng xảy ra b) tính thể tích o2 ở đktc cần dùng c) muốn có được thể tích oxi trên phải phân hủy bao nhiêu gam KClo3
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


1. a. \(PTHH:2Mg+O_2\overset{t^o}{--->}2MgO\left(1\right)\)
b. Ta có: \(n_{Mg}=\dfrac{24}{24}=1\left(mol\right)\)
Theo PT(1): \(n_{O_2}=\dfrac{1}{2}.n_{Mg}=\dfrac{1}{2}.0,1=0,5\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,5.22,4=11,2\left(lít\right)\)
c. \(PTHH:2KClO_3\xrightarrow[t^o]{MnO_2}2KCl+3O_2\left(2\right)\)
Theo PT(2): \(n_{KClO_3}=\dfrac{2}{3}.n_{O_2}=\dfrac{2}{3}.0,5=\dfrac{1}{3}\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=\dfrac{1}{3}.122,5=40,83\left(g\right)\)
2. \(PTHH:3Fe+2O_2\overset{t^o}{--->}Fe_3O_4\)
Ta có: \(n_{Fe_3O_4}=\dfrac{2,32}{232}=0,01\left(mol\right)\)
a. Theo PT: \(n_{Fe}=3.n_{Fe_3O_4}=0,01.3=0,03\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,03.56=1,68\left(g\right)\)
b. Theo PT: \(n_{O_2}=2.n_{Fe_3O_4}=2.0,01=0,02\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,02.22,4=0,448\left(lít\right)\)

\(n_{Fe}=\dfrac{126}{56}=2,25\left(mol\right)\\
pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
2,25 1,5
=> \(V_{O_2}=1,5.22,4=33,6\left(L\right)\)
\(PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
1 1,5
=> \(m_{KClO3}=122,5\left(g\right)\)

a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{Al}=\dfrac{8,1}{27}=0,3\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,225\left(mol\right)\Rightarrow V_{O_2}=0,225.22,4=5,04\left(l\right)\)
c, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,15\left(mol\right)\Rightarrow m_{KClO_3}=0,15.122,5=18,375\left(g\right)\)

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ a,2Mg+O_2\rightarrow\left(t^o\right)2MgO\\ n_{O_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ V_{O_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ b,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\\ n_{KClO_3}=\dfrac{0,05.2}{3}=\dfrac{1}{30}\left(mol\right)\\ \Rightarrow m_{KClO_3}=\dfrac{122,5}{30}=\dfrac{49}{12}\left(g\right)\)

\(n_P=\dfrac{7,44}{31}=0,24mol\)
\(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
0,24 0,3 0,12
\(V_{O_2}=0,3\cdot22,4=6,72l\)
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
0,2 0,3
\(m_{KClO_3}=0,2\cdot122,5=24,5g\)

3Fe+2O2-to>Fe3O4
0,225--0,15
n Fe=\(\dfrac{12,6}{56}\)=0,225 mol
VO2=0,15.22,4=3,36l
2KClO3-to>2KCl+3O2
0,1---------------------0,15
=>m KClO3=0,1.122,5=12,25g
\(a,3Fe+2O_2\rightarrow Fe_3O_4\)
\(b,\)
Ta có : \(n_{Fe}=\dfrac{m}{M}=\dfrac{126}{56}=2,25\left(mol\right)\)
\(\Rightarrow n_{O_2}=\dfrac{2}{3}n_{Fe}=\dfrac{2}{3}.2,25=1,5\left(mol\right)\)
\(\Rightarrow VO_2=33,6\left(l\right)\)
\(c,\)
\(PTHH:2KClO_3\rightarrow2KCl+3O_2\)
Theo \(PTHH:n_{KClO_3}=\dfrac{2}{3}n_{O_2}=\dfrac{2}{3}.1,5=1\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=n.M=1,122,5=122,5\left(g\right)\)

\(n_{Fe}=\dfrac{25,2}{56}=0,45\left(mol\right)\\ a,PTHH:3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\\ b,n_{O_2}=\dfrac{2}{3}.0,45=0,3\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\\ c,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\\ n_{KClO_3}=\dfrac{2}{3}.0,3=0,2\left(mol\right)\\ \Rightarrow m_{KClO_3}=122,5.0,2=24,5\left(g\right)\)

mMg = 3.6/24 = 0.15 (mol)
2Mg + O2 -to-> 2MgO
0.15__0.075____0.15
mMgO= 0.15*40 = 6 (g)
VO2 = 0.075*22.4 = 1.68 (l)
2KClO3 -to-> 2KCl + 3O2
0.05_______________0.075
mKClO3 = 0.05*122.5 = 6.125 (g)
PTHH: \(2Mg+O_2\underrightarrow{t^o}2MgO\)
a+b) Ta có: \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=0,075\left(mol\right)\\n_{MgO}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{O_2}=0,075\cdot22,4=1,68\left(l\right)\\m_{MgO}=0,15\cdot40=6\left(g\right)\end{matrix}\right.\)
c) PTHH: \(2KClO_3\xrightarrow[MnO_2]{t^o}2KCl+3O_2\uparrow\)
Theo PTHH: \(n_{KClO_3}=0,05\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=0,05\cdot122,5=6,125\left(g\right)\)
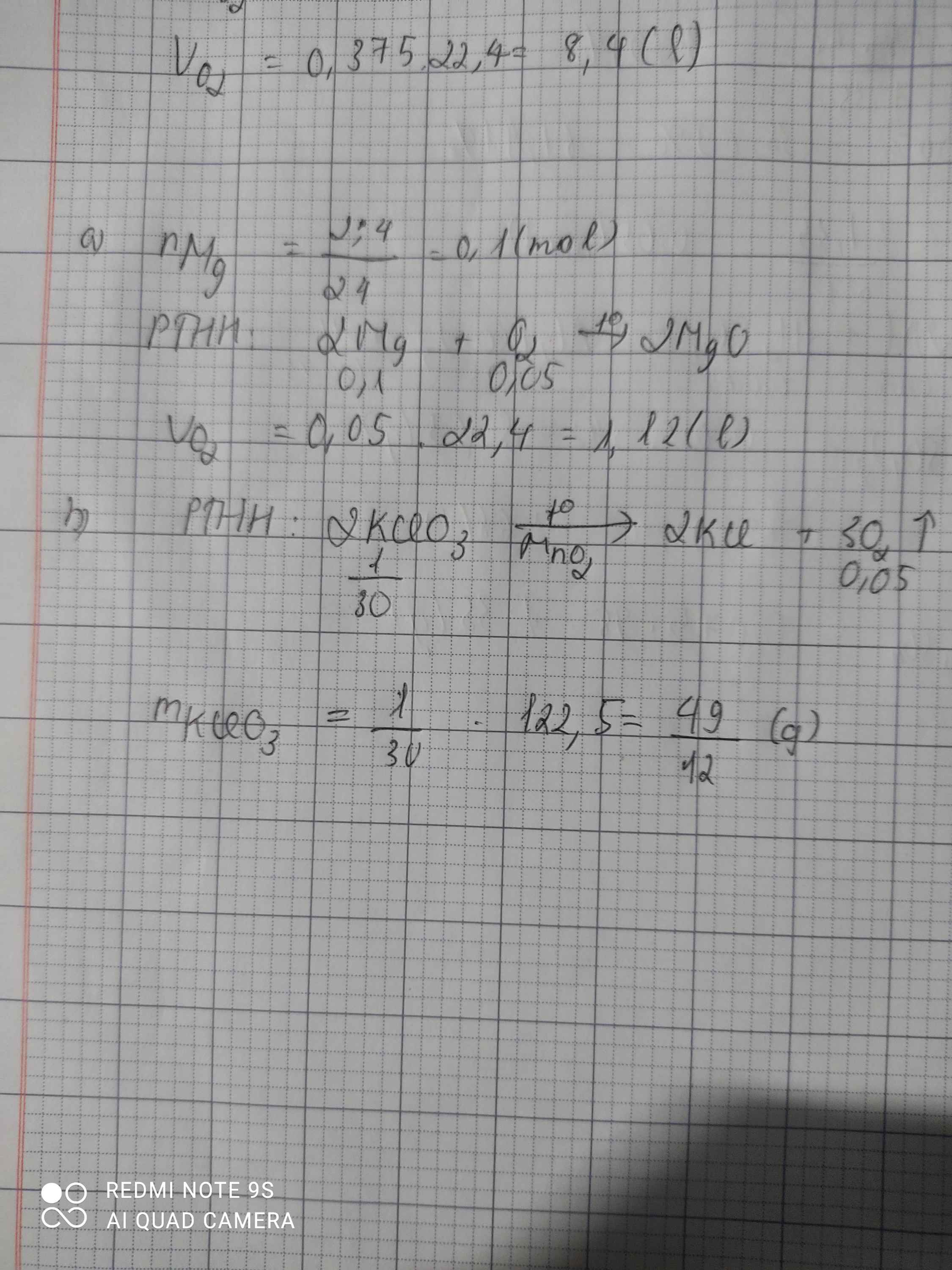
a, PT: \(2Mg+O_2\underrightarrow{t^o}2MgO\)
b, Ta có: \(n_{MgO}=\dfrac{2,4}{40}=0,06\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{MgO}=0,03\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,03.22,4=0,672\left(l\right)\)
c, PT: \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,02\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=0,02.122,5=2,45\left(g\right)\)
Cảm ơn KHÁNH ĐAN rất nhiều