giúp em b3 với ạ cảm ơn trước ạ 
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) \(A=\dfrac{1}{1.3}+\dfrac{1}{3.5}+\dfrac{1}{5.7}+...+\dfrac{1}{19.21}\)
\(A=\dfrac{1}{2}.\left(1-\dfrac{1}{3}+\dfrac{1}{3}-\dfrac{1}{5}+\dfrac{1}{5}-\dfrac{1}{7}+...+\dfrac{1}{19}-\dfrac{1}{21}\right)\)
\(A=\dfrac{1}{2}.\left(1-\dfrac{1}{21}\right)\)
\(A=\dfrac{1}{2}.\left(\dfrac{21}{21}-\dfrac{1}{21}\right)\)
\(A=\dfrac{1}{2}.\dfrac{20}{21}\)
\(A=\dfrac{10}{21}\)
b) \(B=\dfrac{1}{99}-\dfrac{1}{99.98}-\dfrac{1}{98.97}-\dfrac{1}{97.96}-...-\dfrac{1}{3.2}-\dfrac{1}{2.1}\)
\(B=\dfrac{1}{99}-\left(\dfrac{1}{1.2}+\dfrac{1}{2.3}+...+\dfrac{1}{96.97}+\dfrac{1}{97.98}+\dfrac{1}{98.99}\right)\)
\(B=\dfrac{1}{99}-\left(1-\dfrac{1}{2}+\dfrac{1}{2}-\dfrac{1}{3}+...+\dfrac{1}{96}-\dfrac{1}{97}+\dfrac{1}{97}-\dfrac{1}{98}+\dfrac{1}{98}-\dfrac{1}{99}\right)\)
\(B=\dfrac{1}{99}-\left(1-\dfrac{1}{99}\right)\)
\(B=\dfrac{1}{99}-\left(\dfrac{99}{99}-\dfrac{1}{99}\right)\)
\(B=\dfrac{1}{99}-\dfrac{98}{99}\)
\(B=-\dfrac{97}{99}\)


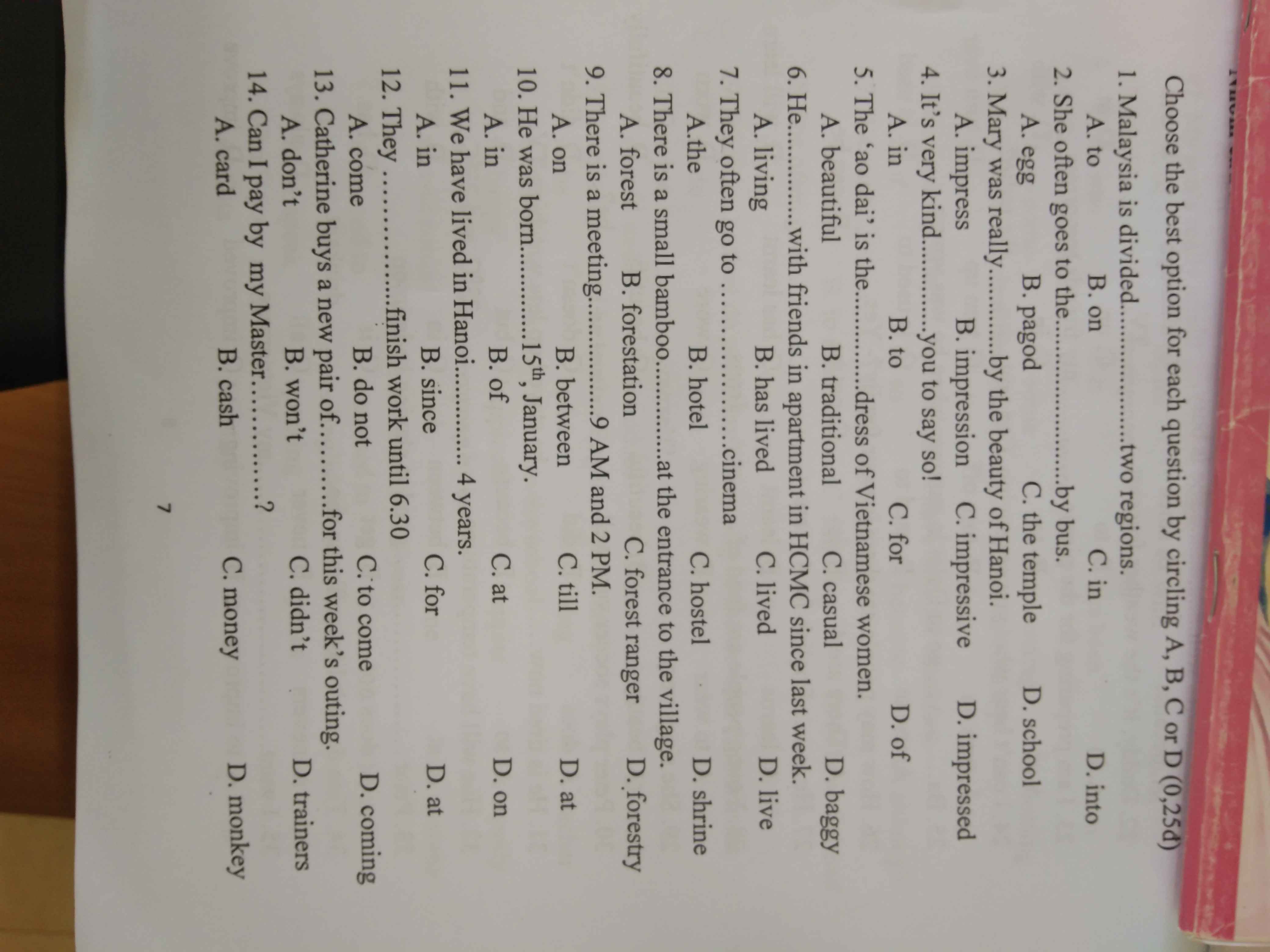
1. What size shoes do you take?
2. What newspaper do you read?
3. What color are your eyes?
4. What time did you arrive this morning?
5. What kind of film do you like?
6. How tall is your teacher?
7. How far is it from your house to the office?
8. How much did you pay for your new shirt?
9. How often do you take an English test in class?
10. How long have you been studying English?





 giúp em với ạ em cảm ơn trước ạ
giúp em với ạ em cảm ơn trước ạ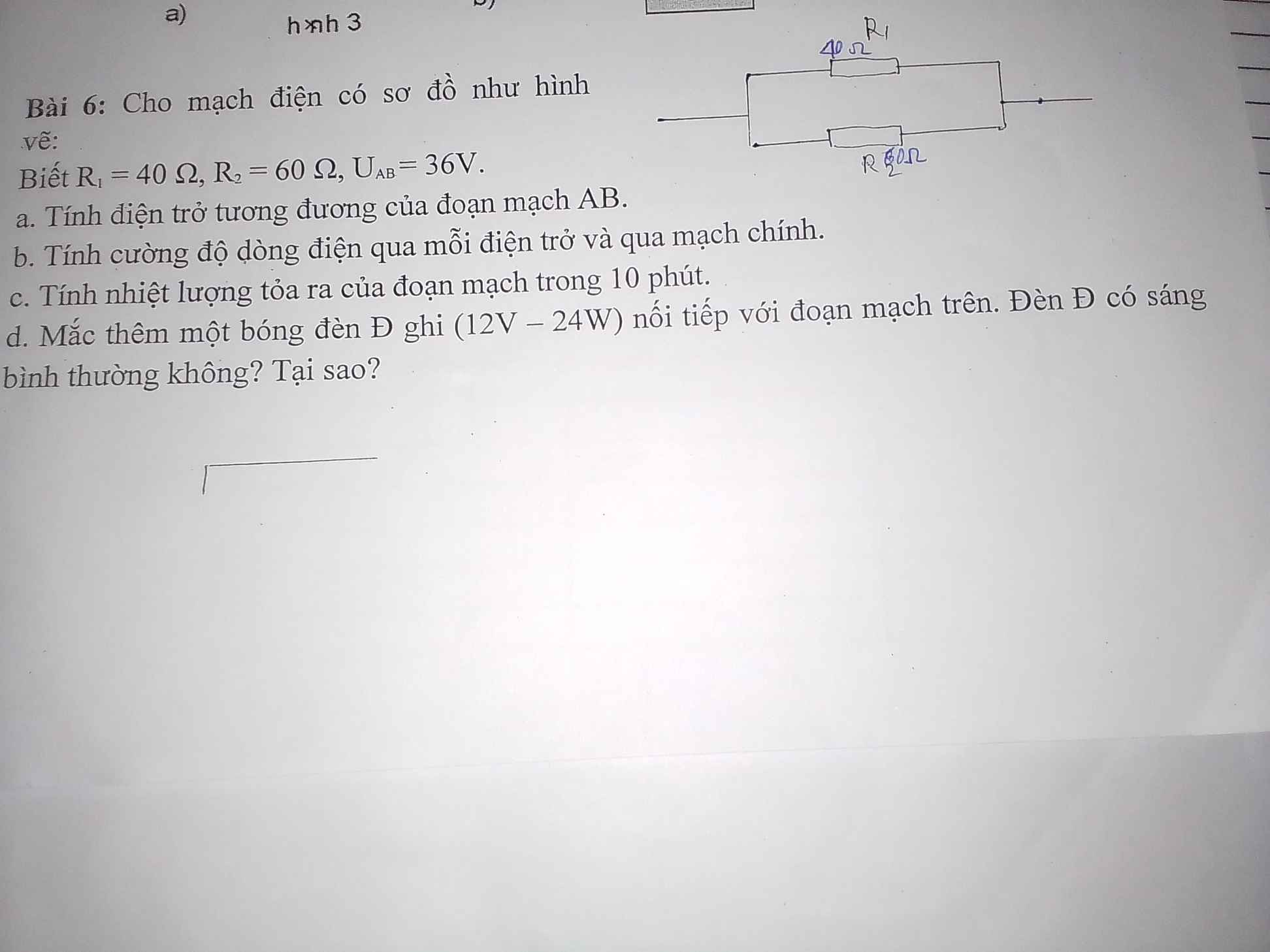








Bài 3:
1) Quy hết hỗn hợp kim loại về kim loại X (hoá trị II)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: \(X+2HCl\rightarrow XCl_2+H_2\)
0,3<----------------------0,3
\(\rightarrow M_X=\dfrac{21,7}{0,3}=72,33\left(g\text{/}mol\right)\)
\(\rightarrow M_R< M_X< M_{Ba}\)
Mà R có hoá trị II và có phản ứng với nước
=> R là Ca
2) Gọi \(\left\{{}\begin{matrix}n_{Ba}=x\left(mol\right)\\n_{Ca}=y\left(mol\right)\end{matrix}\right.\)
\(\rightarrow137x+40y=21,7\left(1\right)\)
Mà \(n_R=n_{Ba}+n_{Ca}\)
\(\rightarrow x+y=0,3\left(2\right)\)
Từ \(\left(1\right),\left(2\right)\rightarrow\left\{{}\begin{matrix}x=0,1\left(mol\right)\\y=0,2\left(mol\right)\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{Ba}=\dfrac{0,1.137}{21,7}.100\%=63,13\%\\\%m_{Ca}=100\%-63,13\%=36,87\%\end{matrix}\right.\)