Mọi người giải hộ em với ạ em đang cần gấp 
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.




a: Xét tứ giác OBAC có
\(\widehat{OBA}+\widehat{OCA}=180^0\)
Do đó: OBAC là tứ giác nội tiếp

IV,
1. No, it wasn't
2. Two French brothers, Louis and Auguste Lumie
3. In the 1927
4. Because of the arrival of television in 1950s
V,
1. Why were the girls very happy?
2. Who are working in the field now ?
3. How long has Mary learned English ?
4. It was not until 2.30 that someone could leave the stadium
5. It was not until the age of 24 that Mary stopped learning Germen
6. It was not until their parents came home that they cleaned their house

\(1,\\ a,\dfrac{8x}{2xy}=\dfrac{4x}{y}\\ b,\dfrac{2xy}{6y}=\dfrac{x}{3}\\ c,\dfrac{3\left(x+2\right)}{2x}=\dfrac{6\left(x+2\right)}{4x}\\ d,\dfrac{4\left(x-2\right)}{3\left(x+1\right)}=\dfrac{8\left(x-2\right)x}{6\left(x+1\right)x}\\ 2,\\ \dfrac{x^2+3x+2}{x^2+x}=\dfrac{x^2+x+2x+2}{x\left(x+1\right)}=\dfrac{\left(x+1\right)\left(x+2\right)}{x\left(x+1\right)}=\dfrac{x+2}{x}\\ 3,\\ \dfrac{x^2-3x}{x^2-9}=\dfrac{x}{x+3}\)

Bài 3:
Ta có: \(x^2-2x+4=\left(x-1\right)^2+3\ge3\forall x\)
\(\Leftrightarrow P=\dfrac{15}{x^2-2x+4}=\dfrac{15}{\left(x-1\right)^2+3}\le5\forall x\)
Dấu '=' xảy ra khi x=1

3:
a:Các tia trên hình là Ax,Ay,Bx,By,Cx,Cy
=>Có 6 tia
b: AB<AC
=>B nằm giữa A và C
=>AB+BC=AC
=>BC=4cm
c: AI=3/2=1,5cm
CI=7-1,5=5,5cm

a) \(\dfrac{A}{x-2}=\dfrac{x^2+3x+2}{x^2-4}\)
\(\Leftrightarrow\dfrac{A}{x-2}=\dfrac{\left(x+2\right)\left(x+1\right)}{\left(x-2\right)\left(x+2\right)}\)
\(\Leftrightarrow\dfrac{A}{x-2}=\dfrac{x+1}{x-2}\Leftrightarrow A=x+1\)
b) \(\dfrac{M}{x-1}=\dfrac{x^2+3x+2}{x+1}\)
\(\Leftrightarrow\dfrac{M}{x-1}=\dfrac{\left(x+1\right)\left(x+2\right)}{x+1}\)
\(\Leftrightarrow\dfrac{M}{x-1}=x+2\Leftrightarrow M=\left(x-1\right)\left(x+2\right)=x^2+x-2\)




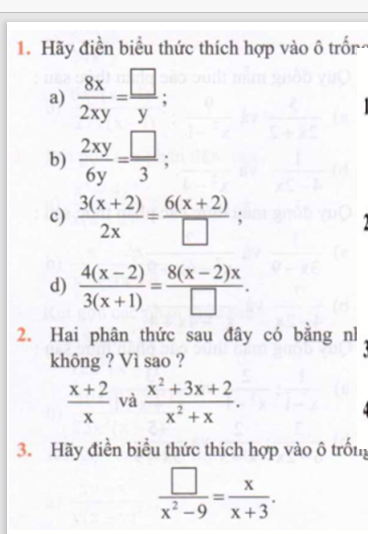 Mọi người giải giúp em với ạ em đang cần gấp ạ
Mọi người giải giúp em với ạ em đang cần gấp ạ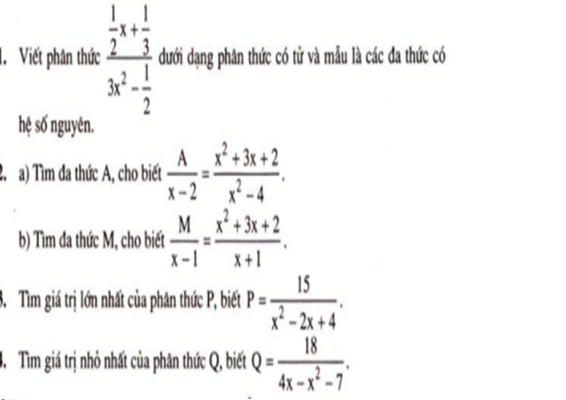 Mọi người giải giúp em với ạ em đang cần gấp ạ
Mọi người giải giúp em với ạ em đang cần gấp ạ
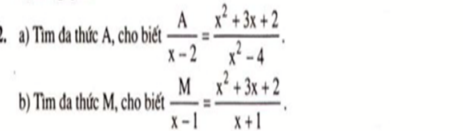 mọi người giải giúp em bài này với ạ em đang cần gấp ạ
mọi người giải giúp em bài này với ạ em đang cần gấp ạ
Câu 3:
a)
CTPT xủa X là CnH2n+2O
\(n_{CO_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\Rightarrow n_{C_nH_{2n+2}O}=\dfrac{0,4}{n}\left(mol\right)\)
=> \(n_{H_2O}=\dfrac{\dfrac{0,4}{n}.\left(2n+2\right)}{2}=\dfrac{0,4}{n}\left(n+1\right)\left(mol\right)\)
Mà \(n_{H_2O}=\dfrac{9}{18}=0,5\left(mol\right)\)
=> n = 4
=> CTPT: C4H10O
b) \(n_{C_4H_{10}O}=\dfrac{0,4}{4}=0,1\left(mol\right)\)
=> m = 0,1.74 = 7,4 (g)
c)
(1) \(CH_3-CH_2-CH_2-CH_2OH\)
(2) \(CH_3-CH_2-CH\left(OH\right)-CH_3\)
(3) \(CH_3-C\left(CH_3\right)\left(OH\right)-CH_3\)
(4) \(CH_3-CH\left(CH_3\right)-CH_2OH\)
(5) \(CH_3-CH_2-CH_2-O-CH_3\)
(6) \(CH_3-CH\left(CH_3\right)-O-CH_3\)
(7) \(CH_3-CH_2-O-CH_2-CH_3\)
d)
X là \(CH_3-C\left(CH_3\right)\left(OH\right)-CH_3\) (2-metylpropan-2-ol)