HCl+KMnO4→Cl2+MnO2+KCl+H2O
tính thể tích khí clo (đktc) thu được 31,6g KMnO4 với HCl đặc dư với hiệu suất phản ứng 80%
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PT: \(2KMnO_4+16HCl\rightarrow2MnCl_2+2KCl+5Cl_2+8H_2O\)
Ta có: \(n_{KMnO_4}=\dfrac{31,6}{158}=0,2\left(mol\right)\)
\(m_{HCl}=\dfrac{60.36,5}{100}=21,9\left(g\right)\Rightarrow n_{HCl}=\dfrac{21,9}{36,5}=0,6\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,2}{2}>\dfrac{0,6}{16}\), ta được KMnO4 dư.
Theo PT: \(n_{Cl_2\left(LT\right)}=\dfrac{5}{16}n_{HCl}=0,1875\left(mol\right)\)
Mà: H% = 80%
\(\Rightarrow n_{Cl_2\left(TT\right)}=0,1875.80\%=0,15\left(mol\right)\)
\(\Rightarrow V_{Cl_2}=0,15.22,4=3,36\left(l\right)\)
Bạn tham khảo nhé!

\(n_{Cl_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
PTHH: \(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
0,04<-----------------------------------------------0,1
\(\Rightarrow n_{KMnO_4\left(c\text{ần}.d\text{ùng}\right)}=\dfrac{0,04}{80\%}=0,05\left(mol\right)\)
\(\Rightarrow m_{KMnO_4}=0,05.158=7,9\left(g\right)\)
PT: \(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
Ta có: \(n_{Cl_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
Theo PT: \(n_{KMnO_4\left(LT\right)}=\dfrac{2}{5}n_{Cl_2}=0,04\left(mol\right)\)
Mà: H% = 80% \(\Rightarrow n_{KMnO_4\left(TT\right)}=\dfrac{0,04}{80\%}=0,05\left(mol\right)\)
\(\Rightarrow m_{KMnO_4}=0,05.158=7,9\left(g\right)\)

\(n_{KMnO_4}=\dfrac{m_{KMnO_4}}{M_{KMnO_4}}=\dfrac{47,4}{158}=0,3mol\)
\(n_{KMnO_4}=\dfrac{0,3}{80\%}=0,375mol\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
2 16 2 2 5 8 ( mol )
0,375 > 2,5 ( mol )
0,375 0,9375 ( mol )
\(V_{Cl_2}=n_{Cl_2}.22,4=0,9375.22,4=21l\)
\(n_{KMnO_4\left(bd\right)}=\dfrac{47,4}{158}=0,3\left(mol\right)\) => \(n_{KMnO_4\left(pư\right)}=\dfrac{0,3.80}{100}=0,24\left(mol\right)\)
PTHH: 2KMnO4 + 16HCl --> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
0,24------------------------------------->0,6
=> \(V=0,6.22,4=13,44\left(l\right)\)

MnO\(_2\)+4HCl\(\rightarrow\)MnCl\(_2\)+Cl\(_2\)+2H\(_2O\)
0,45 0,45 (mol)
n\(_{MnO_2}\)=\(\dfrac{39,15}{87}\)=0,45(mol)
2Fe + 3Cl\(_2\)\(\rightarrow\)2FeCl\(_3\)
0,3 0,45 0,3 (mol)
m\(_{FeCl_3}\)=0,3.162,5=48,75(g)
vì hiệu suất phản ứng là 86% nên:
m\(_{FeCl_3}\)=\(\dfrac{86.48,75}{100}\)=41,925(g)
2/
Mg+Cl\(_2\)\(\rightarrow\)MnCl\(_2\)
0,6 0,6
n\(_{Mg}\)=\(\dfrac{14,4}{24}\)=0,6(mol)
2\(KMnO_4+16HCl\rightarrow2MnCl_2+2KCl+5Cl_2\uparrow+8H_2O\)
0,24 0,6
vì hiệu suất phản ứng bằng 80%,nên để điều chế 0,6 mol Cl\(_2\)thì cần số mol \(KMnO_4\) là:
n\(_{KMnO_4}\)=\(\dfrac{0,24.100}{80}\)=0,3(mol)
m\(KMnO_4\)=0,3.158=47,4(g)

\(n_{MnO_2}=\dfrac{17,4}{87}=0,2\left(mol\right)\\ PTHH:MnO_2+4HCl_{đặc,nóng}\rightarrow MnCl_2+Cl_2+2H_2O\\ n_{Cl_2\left(TT\right)}=\dfrac{3,584}{22,4}=0,16\left(mol\right)\\ n_{Cl_2\left(LT\right)}=n_{MnO_2}=0,2\left(mol\right)\\ \Rightarrow H=\dfrac{n_{Cl_2\left(TT\right)}}{n_{Cl_2\left(LT\right)}}.100\%=\dfrac{0,16}{0,2}.100=80\%\)

- PT: a, \(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\) (1)
\(MnO_2+4HCl_đ\underrightarrow{t^o}MnCl_2+Cl_2+2H_2O\) (2)
- Ta có: \(n_{HCl\left(1\right)}=n_{HCl\left(2\right)}=0,2.2=0,4\left(mol\right)\)
Theo PT (1): \(n_{Cl_2}=\dfrac{5}{16}n_{HCl\left(1\right)}=0,125\left(mol\right)\Rightarrow V_1=0,125.22,4=2,8\left(l\right)\)
(2): \(n_{Cl_2\left(2\right)}=\dfrac{1}{4}n_{HCl\left(2\right)}=0,1\left(mol\right)\Rightarrow V_2=0,1.22,4=2,24\left(l\right)\)

bài 17
nKMnO4=23,7\158=0,15 mol
| 16HCl | + | 2KMnO4 | → | 5Cl2 | + | 8H2O | + | 2KCl | + | 2MnCl2 |

1. Ta có:
\(n_{MgO2}=\frac{174}{87}=2\left(mol\right)\)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
Mà H = 70%
\(\Rightarrow n_{Cl2}=70\%.2=1,4\left(mol\right)\)
\(\Rightarrow m_{Cl2}=1,4.71=99,1\left(g\right)\)
2. \(PTHH:2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
Ta có:
\(n_{Cl2}=\frac{8,96}{22,4}=0,4\left(mol\right)\)
\(n_{KMnO4}=\frac{2}{5}n_{Cl2}=0,16\left(mol\right)\)
\(\Rightarrow m_{KMnO4}=0,16.158=25,28\left(g\right)\)
\(\Rightarrow H=\frac{25,28}{31,6}.100\%=80\%\)
3.
\(n_{HCl}=\frac{91,25.20\%}{36,5}=0,5\left(mol\right)\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}:a\left(mol\right)\\n_{Fe2O3}:b\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow56a+160b=13,6\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
a____2a_____________
\(Fe_3O_4+6HCl\rightarrow2FeCl_3+3H_2O\)
b________6b________________
\(\Rightarrow2a+6b=0,5\)
\(\Rightarrow\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,05\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\frac{0,1.56}{13,6}=41,18\%\\\%m_{Fe3O4}=100\%-41,18\%=58,82\%\end{matrix}\right.\)

Đáp án D
Số mol KMnO 4 là
![]()
Sơ đồ phản ứng:
K Mn + 7 O 4 ⏟ 0 , 3 mol + H Cl - 1 → Mn + 2 Cl 2 + Cl 2 ⏟ V lit 0 + KCl + H 2 O
Các quá trình nhường, nhận electron:
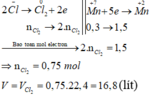
8HCl + 2KMnO4 → 3Cl2 + 2MnO2 + 2KCl + 4H2O
nKMnO4 = 31,6 / 158 = 0,2 (mol)
nCl2 = nKMnO4 * 3/2 * 80% = 0,24 (mol)
→ VCl2 = 0,24 * 22,4 = 5,376 (l)