làm hộ em bài 2 với ạ
làm hộ em bài 2 với ạ
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Y chứa \(\left\{{}\begin{matrix}Al_2\left(SO_4\right)_3:2a\left(mol\right)\\K_2SO_4:a\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{Al^{3+}}=4a\left(mol\right)\\n_{K^+}=2a\left(mol\right)\\n_{SO_4^{2-}}=7a\left(mol\right)\end{matrix}\right.\)
Gọi \(\left\{{}\begin{matrix}n_{Ba^{2+}}=x\left(mol\right)\\n_{OH^-}=2x\left(mol\right)\end{matrix}\right.\)
- Nếu Z chứa K2SO4
Ba2+ + SO42- --> BaSO4
x----->x------------>x
Al3+ + 3OH- --> Al(OH)3
4a-->12a------>4a
=> \(\left\{{}\begin{matrix}n_{K_2SO_4}=n_{SO_4^{2-}\left(còn\right)}=7a-x=0,02\\n_{OH^-}=12a=2x\end{matrix}\right.\)
=> a = 0,02; x = 0,12
=> Y chứa \(\left\{{}\begin{matrix}Al_2\left(SO_4\right)_3:0,04\left(mol\right)\\K_2SO_4:0,02\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{K_2SO_4.Al_2\left(SO_4\right)_3.24H_2O}=0,02\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=0,02\left(mol\right)\end{matrix}\right.\)
=> m1 = 0,02.948 + 0,02.342 = 25,8(g)
m2 = 233.0,12 + 0,08.78 = 34,2 (g)
\(n_{Ba\left(OH\right)_2}=0,12\left(mol\right)\)
=> \(V=\dfrac{0,12}{2}=0,06\left(l\right)=60\left(ml\right)\)
- Nếu Z chứa KAlO2
Ba2+ + SO42- --> BaSO4
x----->x---------->x
Al3+ + 3OH- --> Al(OH)3
4a--->12a----->4a
Al(OH)3 + OH- --> AlO2- + 2H2O
(2x-12a)<-(2x-12a)->(2x-12a)
=> \(\left\{{}\begin{matrix}n_{KAlO_2}=n_{AlO_2^-}=2x-12a=0,02\\n_{KAlO_2}=n_{K^+}=2a=0,02\end{matrix}\right.\)
=> a = 0,01; x = 0,07
=> Y chứa \(\left\{{}\begin{matrix}Al_2\left(SO_4\right)_3:0,02\left(mol\right)\\K_2SO_4:0,01\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{K_2SO_4.Al_2\left(SO_4\right)_3.24H_2O}=0,01\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=0,01\left(mol\right)\end{matrix}\right.\)
=> m1 = 0,01.948 + 0,01.342 = 12,9(g)
Kết tủa gồm \(\left\{{}\begin{matrix}BaSO_4:0,07\left(mol\right)\\Al\left(OH\right)_3:0,02\left(mol\right)\end{matrix}\right.\)
=> m2 = 0,07.233 + 0,02.78 = 17,87 (g)
\(V=\dfrac{0,07}{2}=0,035\left(l\right)=35\left(ml\right)\)
mkhí, hơi = 3,552 - 0,96 = 2,592
\(n_{KOH}=\dfrac{1,344.100}{100.56}=0,024\left(mol\right)\)
mdd sau pư = 100 + 2,592 = 102,592 (g)
Gọi công thức của muối cần tìm là KaX
=> \(n_{K_aX}=\dfrac{0,024}{a}\left(mol\right)\)
Có: \(m_{muối}=\dfrac{2,363.102,592}{100}=2,42425\left(g\right)\)
=> \(M_{K_aX}=39a+M_X=\dfrac{2,42425}{\dfrac{0,024}{a}}\left(g/mol\right)\)
=> MX = 62a (g/mol)
Xét a = 1 => MX = 62 (NO3)
Xét a = 2,3 => Loại
\(n_{KNO_3}=0,024\left(mol\right)\)
Gọi CTHH của muối là A(NO3)n.qH2O
Bảo toàn N: \(n.n_{A\left(NO_3\right)n.qH_2O}=0,024\left(mol\right)\)
Bảo toàn A: \(n_{A\left(NO_3\right)_n.qH_2O}=n_{A_xO_y}=\dfrac{0,96}{x.M_A+16y}\left(mol\right)\)
=> \(n.\dfrac{0,96}{x.M_A+16y}=0,024\)
=> 0,024.x.MA + 0,384y = 0,96n
( Do hóa trị không đổi nên \(n=\dfrac{2y}{x}\))
- Nếu \(n=\dfrac{2y}{x}=1\) => MA = 12 (Loại)
- Nếu \(n=\dfrac{2y}{x}=2\) => MA = 64 (Cu)
- Nếu \(n=\dfrac{2y}{x}=3\) => MA = 36 (Loại)
=> CTHH của muối là Cu(NO3)2.qH2O
\(n_{CuO}=\dfrac{0,96}{80}=0,012\left(mol\right)\)
=> \(n_{Cu\left(NO_3\right)_2.qH_2O}=0,015\left(mol\right)\)
=> \(M_{Cu\left(NO_3\right)_2.qH_2O}=\dfrac{3,552}{0,012}=296\left(g/mol\right)\)
=> q = 6
=> CTHH: Cu(NO3)2.6H2O


Bài 1:
a: =8xy/2x=4y
b: \(=\dfrac{4x-1-7x+1}{3x^2y}=\dfrac{-3x}{3x^2y}=\dfrac{-1}{xy}\)
c: \(=\dfrac{3x-x+6}{2x\left(x+3\right)}=\dfrac{2\left(x+3\right)}{2x\left(x+3\right)}=\dfrac{1}{x}\)
e: \(=\dfrac{5\left(x+2\right)}{4\left(x-2\right)}\cdot\dfrac{-2\left(x-2\right)}{x+2}=\dfrac{-10}{4}=-\dfrac{5}{2}\)


Bài 21
1 are
2 enjoy reading
3 loves reading
4 learns
5 ;oles
6 spends
7 thinks
8talks
9 lasts
10 agree
11 will go
12 will be
13 will buy
14 will be
15 will probably join
16 have
17 will go
18 enjoy
a) Yes, they do
b) Because there are many interesting things
c) They talk about the books
d) Tomorrow
e) they will go home and enjoy their books

Y chứa NaOH, NaAlO2
Gọi số mol NaOH, NaAlO2 trong mỗi phần là x, y (mol)
TN1:
\(n_{HCl}=0,1.1=0,1\left(mol\right)\)
PTHH: NaOH + HCl --> NaCl + H2O
0,1<----0,1
=> x = 0,1 (mol)
TN3: nHCl = 0,75.1 = 0,75 (mol)
PTHH: NaOH + HCl --> NaCl + H2O
0,1--->0,1
NaAlO2 + HCl + H2O --> NaCl + Al(OH)3
y------>y------------------------>y
Al(OH)3 + 3HCl --> AlCl3 + 3H2O
\(\dfrac{0,65-y}{3}\)<-(0,65-y)
=> \(n_{Al\left(OH\right)_3\left(3\right)}=y-\dfrac{0,65-y}{3}=\dfrac{4y-0,65}{3}\left(mol\right)\)
TN2: \(n_{HCl}=1.0,45=0,45\left(mol\right)\)
- Nếu kết tủa không bị hòa tan:
PTHH: NaOH + HCl --> NaCl + H2O
0,1--->0,1
NaAlO2 + HCl + H2O --> NaCl + Al(OH)3
0,35<--0,35-------------------->0,35
Điều kiện: y \(\ge\) 0,35
=> \(n_{Al\left(OH\right)_3\left(2\right)}=0,35\left(mol\right)\)
Do \(n_{Al\left(OH\right)_3\left(2\right)}=3.n_{Al\left(OH\right)_3\left(3\right)}\)
=> \(0,35=4y-0,65\)
=> y = 0,25 (Loại)
=> Kết tủa bị hòa tan 1 phần
PTHH: NaOH + HCl --> NaCl + H2O
0,1--->0,1
NaAlO2 + HCl + H2O --> NaCl + Al(OH)3
y---->y------------------------->y
Al(OH)3 + 3HCl --> AlCl3 + 3H2O
\(\dfrac{0,35-y}{3}\)<--(0,35-y)
=> \(n_{Al\left(OH\right)_3\left(2\right)}=y-\dfrac{0,35-y}{3}=\dfrac{4y-0,35}{3}\left(mol\right)\)
Do \(n_{Al\left(OH\right)_3\left(2\right)}=3.n_{Al\left(OH\right)_3\left(3\right)}\)
=> \(\dfrac{4y-0,35}{3}=4y-0,65\)
=> y = 0,2
Vậy trong Y chứa \(\left\{{}\begin{matrix}NaOH:0,3\left(mol\right)\\NaAlO_2:0,6\left(mol\right)\end{matrix}\right.\)
Bảo toàn Na: nNa = 0,9 (mol)
Bảo toàn Al: nAl = 0,6 (mol)
=> m = 0,9.23 + 0,6.27 = 36,9 (g)
Y chứa NaOH, NaAlO2
Gọi số mol NaOH, NaAlO2 trong mỗi phần là x, y (mol)
TN1:
nHCl=0,1.1=0,1(mol)nHCl=0,1.1=0,1(mol)
PTHH: NaOH + HCl --> NaCl + H2O
0,1<----0,1
=> x = 0,1 (mol)
TN3: nHCl = 0,75.1 = 0,75 (mol)
PTHH: NaOH + HCl --> NaCl + H2O
0,1--->0,1
NaAlO2 + HCl + H2O --> NaCl + Al(OH)3
y------>y------------------------>y
Al(OH)3 + 3HCl --> AlCl3 + 3H2O
0,65−y30,65−y3<-(0,65-y)
=> nAl(OH)3(3)=y−0,65−y3=4y−0,653(mol)nAl(OH)3(3)=y−0,65−y3=4y−0,653(mol)
TN2: nHCl=1.0,45=0,45(mol)nHCl=1.0,45=0,45(mol)
- Nếu kết tủa không bị hòa tan:
PTHH: NaOH + HCl --> NaCl + H2O
0,1--->0,1
NaAlO2 + HCl + H2O --> NaCl + Al(OH)3
0,35<--0,35-------------------->0,35
Điều kiện: y ≥≥ 0,35
=> nAl(OH)3(2)=0,35(mol)nAl(OH)3(2)=0,35(mol)
Do nAl(OH)3(2)=3.nAl(OH)3(3)nAl(OH)3(2)=3.nAl(OH)3(3)
=> 0,35=4y−0,650,35=4y−0,65
=> y = 0,25 (Loại)
=> Kết tủa bị hòa tan 1 phần
PTHH: NaOH + HCl --> NaCl + H2O
0,1--->0,1
NaAlO2 + HCl + H2O --> NaCl + Al(OH)3
y---->y------------------------->y
Al(OH)3 + 3HCl --> AlCl3 + 3H2O
0,35−y30,35−y3<--(0,35-y)
=> nAl(OH)3(2)=y−0,35−y3=4y−0,353(mol)nAl(OH)3(2)=y−0,35−y3=4y−0,353(mol)
Do nAl(OH)3(2)=3.nAl(OH)3(3)nAl(OH)3(2)=3.nAl(OH)3(3)
=> 4y−0,353=4y−0,654y−0,353=4y−0,65
=> y = 0,2
Vậy trong Y chứa {NaOH:0,3(mol)NaAlO2:0,6(mol){NaOH:0,3(mol)NaAlO2:0,6(mol)
Bảo toàn Na: nNa = 0,9 (mol)
Bảo toàn Al: nAl = 0,6 (mol)
=> m = 0,9.23 + 0,6.27 = 36,9 (g)

https://hoc24.vn/cau-hoi/.1685893843618 (hoặc bn vào link này cho nhanh cũng đc, mik giải rồi)


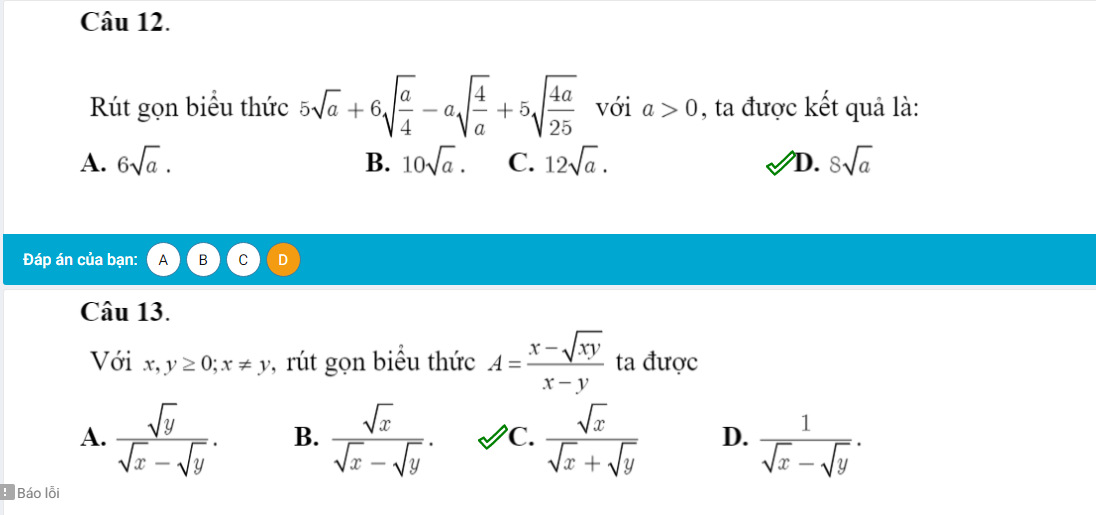
Câu 12.
\(5\sqrt{a}+6\sqrt{\dfrac{a}{4}}-a\sqrt{\dfrac{4}{a}}+5\sqrt{\dfrac{4a}{25}}\)
\(=5\sqrt{a}+6\dfrac{\sqrt{a}}{2}-a\cdot\dfrac{2}{\sqrt{a}}+5\dfrac{2\sqrt{a}}{5}\)
\(=5\sqrt{a}+3\sqrt{a}-2\sqrt{a}+2\sqrt{a}\) (vì a>0)
\(=8\sqrt{a}\)

 Giúp em với ạ
Giúp em với ạ




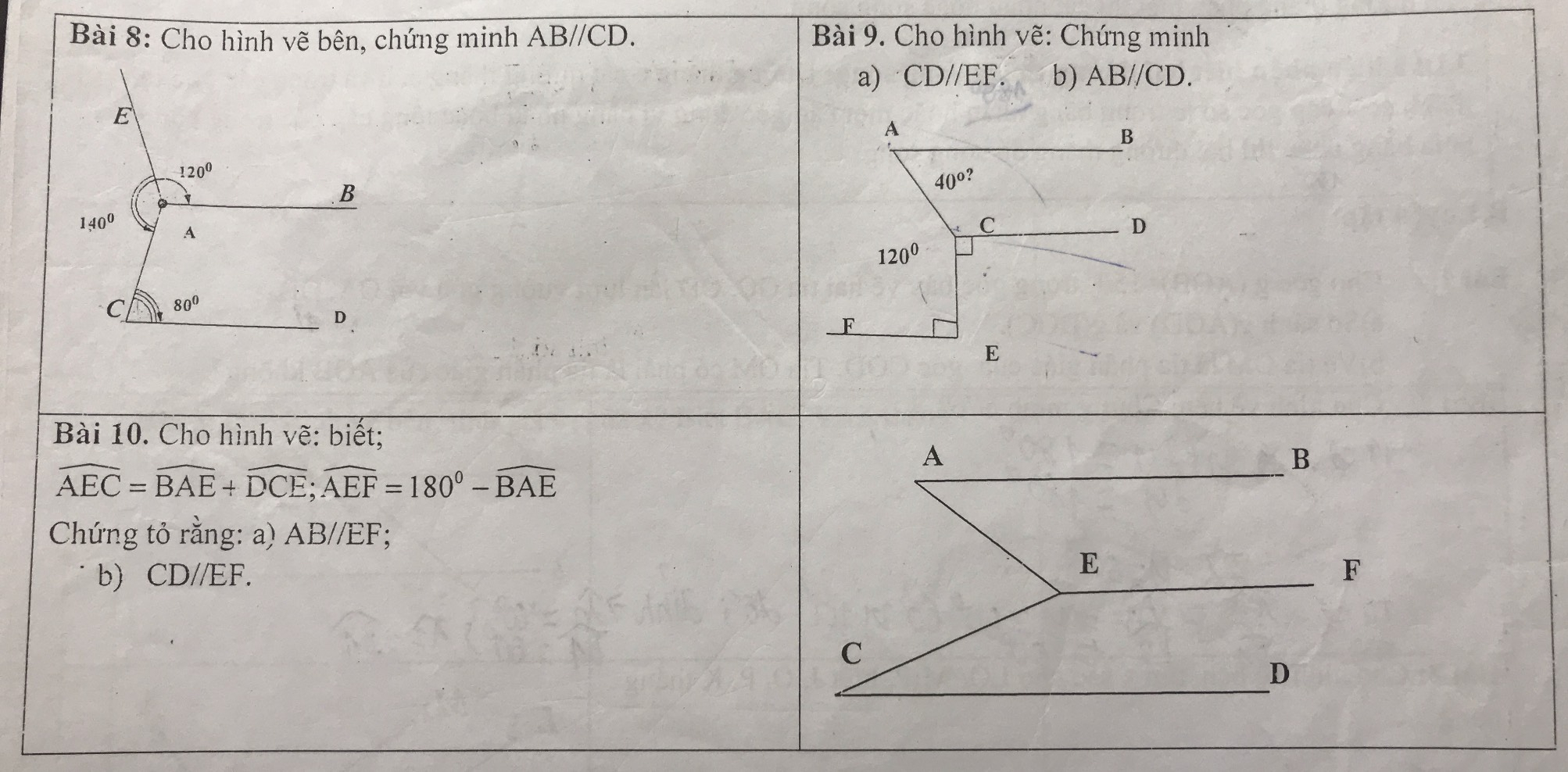 em bài 8,9,10 với ạ em gấp lắm rùi
em bài 8,9,10 với ạ em gấp lắm rùi