Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, Gọi \(m_{NaCl\left(thêm\right)}=a\left(g\right)\)
\(m_{NaCl\left(bđ\right)}=5\%.100=5\left(g\right)\\ \Rightarrow C\%_{NaCl}=\dfrac{5+a}{100+a}.100\%=5,5\%\\ \Leftrightarrow a=0,53\left(g\right)\)
b, \(m_{NaCl}=58,5.5,5\%=3,2175\left(g\right)\\ n_{NaCl}=\dfrac{3,2175}{58,5}=0,055\left(mol\right)\)
PTHH: NaCl + AgNO3 ---> AgCl↓ + NaNO3
0,055-->0,055------>0,055---->0,055
\(m_{AgCl}=0,055.143,5=7,8925\left(g\right)\\ m_{ddY}=58,5+200-7,8925=250,6075\left(g\right)\\ \Rightarrow C\%_{NaNO_3}=\dfrac{0,055.85}{250,6075}.100\%=1,87\%\)

a) MH2+2AgNO3 ->M(NO3)2+2AgH
Fe+MH2 -> FeH2+M
gọi x là số mol của MH2 ở mỗi phần
x(M-56)=0,16=>x=0,16/(M-56)
=>nAgH=0,32/(M-56)
Ta có
mAgH=5,74=>0,32x(108+H)/(M-56)=5,74
=>(108+H)/(M-56)=17,9375
=>17,9375M-H=1112,5
thay H lần lượt là Cl , Br và I ta có
H là Cl thì M là Cu
=>CTHH của X là CuCl2
b)
ta có x(64-56)=0,16=>x=0,02 mol
=>mCuCl2=0,02x2x135=5,4 g

PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
\(FeSO_4+Ba\left(OH\right)_2\rightarrow BaSO_4\downarrow+Fe\left(OH\right)_2\downarrow\)
Ta có: \(n_{H_2SO_4}=0,3\cdot0,5=0,15\left(mol\right)=n_{Fe}=n_{H_2}=n_{Ba\left(OH\right)_2}\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,15\cdot56=8,4\left(g\right)\\V_{H_2}=0,15\cdot22,4=3,36\left(l\right)\\V_{Ba\left(OH\right)_2}=\dfrac{0,15}{1}=0,15\left(l\right)=150\left(ml\right)\end{matrix}\right.\)
*Bạn xem lại đề vì nếu FeSO4 p/ứ hết thì sẽ có nhiều hơn 41,7 gam kết tủa

The Eiffel Tower is located in Paris, France. It was constructed between 1887 and 1889 as anentrance way to the 1889 World’s Fair and to celebrate the 100thanniversary of the French Revolution. The Tower was opened to visitors on May 6th, 1889.
Gustave Eiffel’s design was chosen from among 107 designs that were submitted to the World’s Fair design competition. However, many Parisians, especially artists, did not like his design and protested the tower’s construction. They thought it would be an eyesore, but once it was built, most Parisians soon loved the tower.
The tower is made of iron and weighs over 10,000 tons. It is 324 meters tall, including an antenna at its top, and has a staircase with 1,665 steps. There are also elevators to take visitors to the top platform where there is a panoramic view of Paris. The original elevators, now computerized, are still in use. Over 60 tons of paint are applied to the tower every seven years to keep it from rusting.
The Eiffel Tower has become a symbol of Paris. It is the most recognized monument in Europe mainly because many people think it is an architectural masterpiece. Over 250 million people have visited it since May of 1889.
70.66. How long did it take to build The Eiffel Tower?(1 Point)A. around 2 years B. around 6 years C. 100 yearsD. 60 years
71.67. Who designed The Eiffel Tower? (1 Point)A. the Parisians B. the French RevolutionC. Gustave EiffelD. the World’s Fair
72.68. Why do they repaint The Eiffel Tower frequently?(1 Point)A. to keep it from rustingB. to make it more beautifulC. to attract visitorsD. to display it in the World’s Fair design competition
73.69. How many people have visited The Eiffel Tower until now?(1 Point)A. about 10.000 peopleB. about 324 million peopleC. more than 250 million peopleD. about 189 million people
74.70. What is the major reason for The Eiffel Tower to be a well-known tourist attraction in Europe?(1 Point)A. Because of its history.B. Because it is computerized.C. Because it has architectural beauty.D. Because it is repainted frequently.

Ở $20^oC$,
300 gam nước hòa tan 108,6 gam NaCl thu được dung dịch bão hòa
Suy ra :
100 gam nước hòa tan x gam NaCl thu được dung dịch bão hòa
$\Rightarrow x = \dfrac{100.108,6}{300} = 36,2(gam)$
$\Rightarrow S_{NaCl} = 36,2(gam)$
b)
$S_{AgNO_3} = 222(gam)$, tức là :
222 gam $AgNO_3$ tan tối đa trong 100 gam nước tạo thành dung dịch bão hòa
Suy ra:
y gam $AgNO_3$ tan tối đa trong 250 gam nước tạo thành dung dịch bão hòa
$\Rightarrow y = \dfrac{250.222}{100} = 2,50222(gam)$

\(PTHH:NaCl+AgNO_3\rightarrow NaNO_3+AgCl\downarrow\)
Ta có :
\(n_{AgCl\downarrow}=\frac{129,15}{143,5}=0,9\left(mol\right)\)
Theo phương trình :
\(n_{NaCl}=n_{AgCl\downarrow}=0,9\left(mol\right)\)
\(\Rightarrow m_{NaCl}=0,9.58,5=52,65\left(g\right)\)
Theo đề bài 250g dung dịch NaCl có :
\(\frac{250.36}{136}=66,2\left(g\right)>52,65\left(g\right)\)
Vậy dung dịch chưa bão hòa
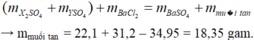
\(\left\{{}\begin{matrix}n_{NaCl}=x\left(mol\right)\\n_{KCl}=y\left(mol\right)\end{matrix}\right.\)
\(n_{AgNO_3}=\dfrac{13,6}{170}=0,08mol\)
\(n_{AgCl\downarrow}=\dfrac{9,471}{143,5}=0,066mol\)
\(AgNO_3+NaCl\rightarrow AgCl\downarrow+NaNO_3\)
x x
\(AgNO_3+KCl\rightarrow AgCl\downarrow+KNO_3\)
y y
Mà \(\Sigma n_{AgCl}=x+y=0,066mol\)
\(\Sigma n_{AgNO_3}=x+y=0,08>\Sigma n_{AgCl}\)
\(\Rightarrow AgNO_3\) dư và dư 0,08-0,066=0,014mol
Thanks